| |
Comparison of Rosiglitazone and Metformin for Treating HIV Lipodystrophy: results suggest improvements in lipoatrophy associated with rosiglitazone.
|
| |
| |
Annals of Internal Medicine
6 September 2005
Jeroen P.H. van Wijk, MD; Eelco J.P. de Koning, MD, PhD; Manuel Castro Cabezas, MD, PhD; Jos op't Roodt, BSc; Jorge Joven, MD, PhD; Ton J. Rabelink, MD, PhD; and Andy I. Hoepelman, MD, PhD
From University Medical Center, Utrecht, the Netherlands; Leiden University Medical Center, Leiden, the Netherlands; St. Franciscus Gasthuis, Rotterdam, the Netherlands; and Centre de Recerca Biomèdica, Hospital Universitari de Sant Joan, Reus, Spain.
People have remarked to me that they see improvement in my lipoatrophy. I cannot say exactly what the improvement is due to, but I have been on pioglitazone for about 1 year. Jules Levin
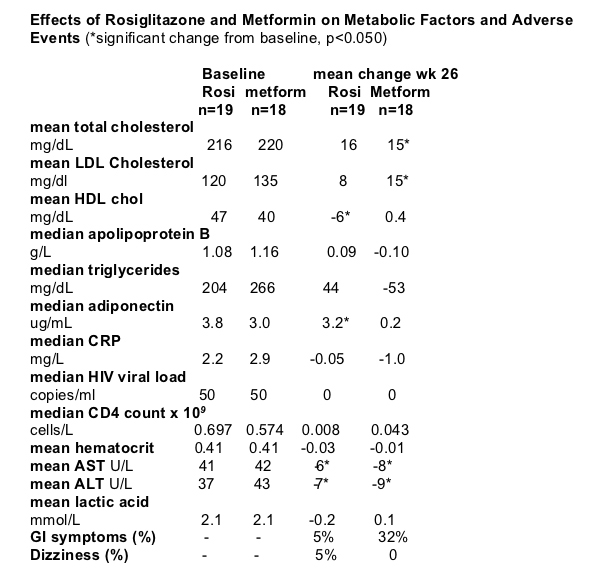
Summary Note from Jules Levin: Patients were all men in this study. As you can see immediately below regarding the effects on body fat distribution, rosiglitazone had statistically significant increases in median body weight, median total body fat, mean subcutaneous abdominal fat, and 9 patients (47%) taking rosiglitazone reported subjective improvement in lipodystrophy, which was corroborated by clinical exam in most of the patients. Increase in subcutaneous fat by rosiglitazone was related to high insulin AUC at baseline suggesting that patients with marked lipoatrophy & insulin resistance may benefit the most from rosiglitazone treatment. In this study patients receiving d4T & rosiglitazone did not show an increase in subcutaneous adipose tissue, suggesting that the use of d4T may hinder rosiglitazone-induced improvement in lipoatrophy, which may explain the lack of efficacy in previous studies by Carr & Sutinen that had a baseline imbalance and overuse of stavudine in the rosiglitazone group. However, Metformin had statistically significant decreases in median body weight, median waist circumference, mean waist-to-hip ratio, median total body fat, mean subcutaneous abdominal fat, and mean visceral abdominal fat. Metformin may not be appropriate for patients with lipoatrophy. Regarding lipids (see table just below), cholesterol nonsignificantly increased for both drugs; triglycerides nonsignificantly increased for rosi by 20% but nonsignificantly decreased by 20% for metformin. Regarding adverse events, metformin was associated with GI symptoms (32%), none for rosi. Pioglitazone does not appear to be associated with increases in cholesterol and triglycerides. Although AST/ALT did not increase for patients in this study AST/ALT were normal at baseline, caution should be taken in patients with renal or liver disease or elevated lactic acid levels, which were exclusion criteria in our study. In these patients, treatment with metformin may not be recommended. Among HIV-negative adults, hyperinsulinemia and glucose intolerance are independent predictors of cardiovascular disease. Therefore, the reductions in postchallenge glucose and insulin levels in this study with metformin and rosiglitazone may result in an improved cardiovascular risk profile.
RESULTS
No patient reported changes in dietary pattern and physical exercise during the study, while all participants reported adequate treatment adherence.
Body Fat Distribution
Compared with metformin, rosiglitazone statistically significantly increased subcutaneous and visceral abdominal fat. Figure 3 shows the individual changes in subcutaneous and visceral abdominal fat with rosiglitazone and metformin. Compared with baseline, rosiglitazone increased subcutaneous abdominal fat, while metformin decreased subcutaneous and visceral abdominal fat (Figure 3). The increase in subcutaneous abdominal fat by rosiglitazone was negatively related to body weight at baseline (r = -0.50; P = 0.003) and positively related to the insulin AUC at baseline (r = 0.43; P = 0.010). In the patients receiving stavudine treatment (n = 4), rosiglitazone did not increase subcutaneous abdominal fat. Nine patients (47%) in the rosiglitazone group and 4 patients (22%) in the metformin group reported subjective improvement of lipodystrophy, which was corroborated by clinical examination in most of these patients.
Median body weight.
Rosiglitazone (n=19): mean change at week 26; increased by 2.3 kg (0.8 to 3.2) from 75.7 kg at baseline (p<0.050). metformin: 78.9 kg at baseline, decreased by -1.2 kg (p<0.050).
Median total body fat mass.
Rosiglitazone: mean change at week 26; increased by 2.9 kg (1.1 to 4.3) from 10.3 kg (7.1 to 12.2) at baseline ((p<0.050). Metformin: decreased by -1.4 kg (2.3 to -0.2), (p<0.050).
Mean subcutaneous abdominal fat.
Rosiglitazone: mean change at week 26; increased by 16 cm2 from 98 (3 to 300) at baseline (p<0.050). Metformin: mean change; -11 (p<0.050).
Mean visceral abdominal fat.
Rosiglitazone: mean change at week 26; decreased by -1 cm2 from 158 cm2 (40 to 400) at baseline (ns). Metformin: decreased by -25 cme from 189 cm2 at baseline (p<0.050).
Median waist circumference.
Rosiglitazone: mean change at week 26=0 cm (-1 to 1) from 92 at baseline (84 to 96) (ns). Metformin: mean change at week 26; decreased -3.5 cm (-6.8 to -1.0) (p<0.050).
Metabolic Variables
Rosiglitazone and metformin similarly decreased the AUCs for glucose and insulin. For glucose, the mean change per 2 hours was -1.9 mmol/L (SD, 1.8) (-34.2 mg/dL [SD, 32.4]) (P = 0.040) and -1.1 mmol/L (SD, 0.7) (-19.8 mg/dL [SD, 12.6]) (P = 0.050), respectively. For insulin, the mean change per 2 hours was -258 pmol/L (SD, 294) and -231 pmol/L (SD, 264), respectively (P = 0.01 for each comparison). Fasting free fatty acids levels tended to be lower after treatment with rosiglitazone (P = 0.080). However, the course of free fatty acids after the glucose load remained similar after treatment with either rosiglitazone or metformin. Compared with metformin, rosiglitazone markedly increased adiponectin levels. Metformin showed greater benefits on fasting lipid profile than rosiglitazone. Serum aminotransferase levels, which may indicate the presence of steatosis when elevated (34), decreased in both treatment groups. We did not observe any effect on plasma CRP levels with either rosiglitazone or metformin.
Vascular Measurements
At baseline and at the end of the study, lumen diameter of the brachial artery did not differ between the groups (data not shown). Before treatment, flow-mediated vasodilation was similar in the rosiglitazone group (mean, 4.5% [SD, 2.2%]) and the metformin group (mean, 4.0% [SD, 2.6%]). Compared with baseline, flow-mediated vasodilation increased statistically significantly with metformin (mean change, 1.5% [SD, 0.8%] [95% CI, 0.4% to 3.3%]) but not with rosiglitazone (mean change, 0.7% [SD, 1.2%] [CI, -1.1% to 2.7%]) (Figure 4). When directly compared with rosiglitazone, metformin did not increase flow-mediated vasodilation in the fasting state (between-treatment change from baseline, 0.8% [SD, 1.0%] [CI, -1.1% to 2.8%]) but increased flow-mediated vasodilation 2 hours after glucose (between-treatment change from baseline, 1.2% [SD, 0.6%] [CI, 0.05% to 2.4%]). Metformin and rosiglitazone did not change endothelium-independent vasodilation (data not shown).
AUTHOR DISCUSSION
Lipodystrophy is a major clinical health problem in HIV-infected patients (2-4). We compared the effects of rosiglitazone and metformin for treating HIV lipodystrophy. Our findings emphasize the importance of individualized care in HIV-infected patients. Although rosiglitazone may partly correct lipoatrophy, metformin improves visceral adiposity, lipid profile, and endothelial function.
In our patient groups, waist-to-hip ratio and visceral abdominal fat were high, but subcutaneous abdominal fat was low, consistent with the presence of lipodystrophy in both study groups. Rosiglitazone statistically significantly increased subcutaneous abdominal fat in patients with HIV lipodystrophy, despite ongoing HAART. Whether the outcomes are similar with long-term rosiglitazone therapy remains to be shown. The increase in subcutaneous abdominal fat by rosiglitazone was similar in magnitude to that observed in studies of switching antiretroviral regimens (35, 36). Subjective improvement of lipodystrophy was reported in almost half of the rosiglitazone-treated patients and was corroborated by clinical examination.
Our results are similar to those observed by Hadigan and colleagues (17). However, in Carr and colleagues' study (18), rosiglitazone for 48 weeks did not improve lipoatrophy in a large patient group. Similarly, rosiglitazone did not improve lipoatrophy in a smaller study by Sutinen and colleagues (19). Identifying patients who are most likely to benefit from rosiglitazone treatment is clinically relevant. In our study, the increase in subcutaneous abdominal fat by rosiglitazone was related to low body weight and high insulin AUC at baseline, suggesting that patients with marked lipoatrophy and insulin resistance may benefit the most from rosiglitazone treatment. Stavudine, which was used by only 4 rosiglitazone-treated patients in our study, may hinder rosiglitazone-induced improvement of lipoatrophy. In our study, the patients receiving stavudine therapy did not show an increase in subcutaneous adipose tissue with rosiglitazone, which may explain the lack of efficacy in previous studies that had a baseline imbalance and overuse of stavudine in the rosiglitazone group (18, 19).
In our study, as well as in other published reports (17-19), we observed a detrimental effect on plasma lipid levels with rosiglitazone in some patients with lipodystrophy. Rosiglitazone should, therefore, be used with caution in patients with HIV lipodystrophy and possibly should be avoided in patients who are already hyperlipidemic or given in conjunction with lipid-lowering agents. Metformin reduced subcutaneous and visceral abdominal fat, a finding that has been previously reported in HIV-infected patients (14-16). Hence, metformin may be best for viscerally obese, overweight, dyslipidemic patients and might not be appropriate for patients with predominant lipoatrophy since they may have a further loss of subcutaneous fat. Rosiglitazone increased subcutaneous abdominal fat, an important benefit among patients with lipoatrophy, as shown previously by Hadigan and colleagues (17). Clearly, these findings emphasize the importance of individualized treatment in HIV-infected patients.
Therapeutic modulation of insulin resistance may become an important aspect in the management of HIV-infected patients (37). The treatment doses in our study were somewhat higher than those used in previous studies in HIV-infected patients (14, 15, 17) but were similar to those used in other studies (18, 19). In our study, metformin and rosiglitazone showed similar benefits on postchallenge glucose and insulin levels, despite different mode of actions (11, 12). Our patients were all men and were not particularly hyperinsulinemic. Whether the results can be extrapolated to women or to a more hyperinsulinemic group remains to be shown. The 2 metformin-treated patients who discontinued the study probably had a minor effect on the results, as revealed by a sensitivity analysis (data not shown). We did not find adverse events or drug interactions that might limit long-term use of these agents. However, caution should be taken in patients with renal or liver disease or elevated lactic acid levels, which were exclusion criteria in our study. In these patients, treatment with metformin may not be recommended.
In our study, fasting free fatty acid levels tended to be lower after treatment with rosiglitazone, as previously reported by Hadigan and colleagues (17). Besides improved free fatty acid storage, rosiglitazone may also improve insulin sensitivity indirectly by altered adipocytokine release (12, 17, 18). Levels of adiponectin, an adipocytokine, are reduced in patients with HIV lipoatrophy and insulin resistance, possibly secondary to therapy with nucleoside reverse transcriptase inhibitors (38, 39). Moreover, low adiponectin levels have been associated with a moderately increased cardiovascular disease risk in men with diabetes (40). Of interest, we observed a marked increase in adiponectin level with rosiglitazone but not with metformin, despite reductions in the AUC for insulin with both agents. In our opinion, the increase in adiponectin level may be explained by improved adipocyte function by rosiglitazone and may convey increased protection from atherosclerosis.
Insulin resistance in patients with HIV lipodystrophy can be associated with hepatic steatosis and increased aminotransferase levels (41, 42). We found a decrease in aminotransferase levels not only in the rosiglitazone group, as observed previously (19), but also in the metformin group, which may suggest that both drugs have a beneficial effect on hepatic inflammation and steatosis. However, this remains speculative since we did not directly assess hepatic fat content.
Among HIV-negative adults, hyperinsulinemia and glucose intolerance are independent predictors of cardiovascular disease (43-45). Therefore, the reductions in postchallenge glucose and insulin levels with metformin and rosiglitazone may result in an improved cardiovascular risk profile. Previously, metformin also reduced markers of fibrinolysis in HIV-infected patients (46). So far, previous studies have not examined whether modulation of insulin resistance translates into vascular benefit in HIV. At baseline, the total group of HIV-infected patients had 2-fold impaired endothelial function compared with 15 age-matched healthy men (data on file). In our study, only metformin improved fasting and postprandial endothelial function. Whether this improvement is sufficient to produce clinical benefit is an open issue, but it might be relevant. Previously, pravastatin did not improve endothelial function in a similar study population (24).
Our results of endothelial function contrast with those of a recent study that suggested a greater beneficial effect on endothelial function with rosiglitazone in comparison with metformin in patients with type 2 diabetes (26), possibly due to some additional anti-inflammatory effect (47). Apparently, this finding in patients with diabetes cannot simply be extrapolated to HIV-infected patients. Several factors may explain this discrepancy. First, atherosclerosis is considered a low-grade inflammatory disease. Plasma CRP is a marker of inflammation that predicts future cardiovascular events (48). Unexpectedly, rosiglitazone did not change CRP levels in our study, while previous studies in patients with type 2 diabetes have shown substantial reductions in CRP level with rosiglitazone (49, 50). Presumably, it may be difficult for rosiglitazone to exert substantial effects on inflammation in HIV-infected patients, although levels of adiponectin (which has anti-inflammatory properties) increased with rosiglitazone. Second, the study sample was receiving HAART, which may directly affect endothelial function. Finally, metformin showed greater benefits on lipid profile than rosiglitazone.
Our study has several limitations. The study was not blinded or placebo-controlled and did not measure clinical outcomes. We included participants on the basis of subjective criteria for lipodystrophy, while anthropometric or metabolic inclusion criteria were absent. Also, we did not measure peripheral subcutaneous fat. We found some minor differences in baseline characteristics between groups, which were generally due to few extreme values. Finally, we conducted many statistical tests, and results should be interpreted with caution.
In conclusion, our findings reinforce the importance of individualized care in HIV-infected patients. Although rosiglitazone may partly correct lipoatrophy, metformin improves visceral adiposity, lipid profile, and vascular function.
ARTICLE TEXT
Endothelial dysfunction is an early marker of atherosclerosis and can be assessed clinically by ultrasonography assessment of brachial artery flow-mediated vasodilation. Flow-mediated vasodilation is correlated with degree of atherosclerosis (20), and impaired flow-mediated vasodilation is an independent predictor of future cardiovascular events (21-23). In HIV-infected patients, the use of protease inhibitors has been linked to endothelial dysfunction (7). However, pravastatin did not improve flow-mediated vasodilation in HIV-infected patients, despite reductions in atherogenic lipoprotein levels (24). Rosiglitazone and metformin improve endothelial function (25-27) in HIV-negative individuals, but the effects of either agent in HIV-infected patients are not known.
The researchers recruited men from an HIV clinic in the Netherlands who self-reported fat loss in their face, arms, legs, and buttocks. They received sugar- and fat-loading tests and blood tests to measure levels of insulin, glucose, lipids, and adiponectin. (Adiponectin is a hormone produced by fat cells that affects metabolism of sugar and lipids. High levels are associated with decreased risk for heart attacks.) The researchers measured the amount of fat in the men's bellies with computed tomography. They also measured the ability of an artery in the arm to relax and expand in response to increased blood flow (flow-mediated vasodilation). After these measurements, men were randomly assigned to receive either rosiglitazone or metformin for 6 months. The researchers then repeated the tests and compared how they had changed with the 2 treatments. 39 HIV-infected men with lipodystrophy who had been receiving antiviral drugs for at least 18 months.
Highly active antiretroviral therapy (HAART) in patients with HIV infection has greatly reduced AIDS-related mortality (1) but is associated with changes in fat distribution (lipodystrophy), including peripheral fat loss and central fat accumulation (2-4). Severe forms of lipodystrophy are a major cosmetic concern and can lead to suboptimal adherence to HAART. In addition, lipodystrophy is associated with metabolic risk factors, including insulin resistance and dyslipidemia (2-6), which have been correlated to surrogate markers of cardiovascular disease (7-9). As survival of patients with HIV infection increases, cardiovascular disease could become an important complication (10).
Metformin and rosiglitazone are used in clinical medicine to improve glycemic control in patients with type 2 diabetes (11, 12). However, these agents may also be useful in nondiabetic patients with insulin resistance. Metformin acts mainly by decreasing hepatic insulin resistance and glucose output (11). Rosiglitazone is an agonist for peroxisome proliferator-activated receptor-{gamma} activation, thereby directly influencing the transcription of genes that regulate glucose and lipid metabolism (12). Peroxisome proliferator-activated receptor-{gamma} is preferentially expressed in adipose tissue, and the improvement of insulin resistance is partly secondary to enhanced fatty acid storage in subcutaneous adipocytes and improved adipocyte function, as reflected by the altered secretion of adiponectin (12). In vitro, rosiglitazone promotes adipogenesis, even in the presence of a protease inhibitor (13). Although metformin and rosiglitazone have been investigated separately (14-19), no studies were available that directly compared the effects of rosiglitazone and metformin for treating HIV lipodystrophy.
We conducted a randomized study to compare the effects of rosiglitazone and metformin on insulin sensitivity, body fat distribution, and endothelial function in patients with HIV lipodystrophy.
ABSTRACT
Background: The use of antiretroviral combination therapy in HIV has been associated with lipodystrophy and cardiovascular risk factors.
Objective: To compare the effects of the peroxisome proliferator-activated receptor-{gamma} agonist rosiglitazone and metformin for treating HIV lipodystrophy.
Design: An open, randomized, 6-month clinical trial.
Setting: University Medical Center, Utrecht, the Netherlands.
Patients: 39 HIV-infected men with lipodystrophy.
Intervention: Rosiglitazone, 8 mg/d, or metformin, 2 mg/d.
Measurements: Insulin sensitivity estimated by the oral glucose tolerance test, subcutaneous and visceral abdominal fat measured by single-slice computed tomography, endothelial function measured by flow-mediated vasodilation, and fasting plasma measurements. Two patients in the metformin group withdrew from the study. Complete case analysis was performed.
Results:
Compared with metformin, rosiglitazone increased subcutaneous abdominal fat (between-treatment change from baseline, 27 cm2 [95% CI, 7 cm2 to 46 cm2]) and visceral abdominal fat (between-treatment change from baseline, 24 cm2 [CI, 6 cm2 to 51 cm2]).
The area under the curve for insulin after the oral glucose tolerance test decreased similarly with both agents, but only rosiglitazone increased adiponectin levels.
Metformin showed greater benefits on fasting lipid profile than rosiglitazone.
Flow-mediated vasodilation statistically significantly increased with metformin (mean change, 1.5% [CI, 0.4% to 3.3%]) and not with rosiglitazone (mean change, 0.7% [CI, -1.1% to 2.7%]).
The metformin versus rosiglitazone increases did not statistically differ. Rosiglitazone and metformin did not change C-reactive protein levels.
Limitations: This small trial was not blinded or placebo-controlled and did not measure clinical outcomes.
Conclusions: The findings emphasize the importance of individualized care in HIV-infected patients. Although rosiglitazone may partly correct lipoatrophy, metformin improves visceral fat accumulation, fasting lipid profile, and endothelial function.
Patients
We recruited men between 18 and 70 years of age with documented HIV infection from the Department of Infectious Disease of the University Medical Center, Utrecht, the Netherlands, between March 2003 and January 2004. At the HIV clinic, approximately 600 patients are currently treated for HIV infection, of whom an estimated 25% have clinical signs of lipodystrophy. Inclusion criteria were HIV RNA values less than 10 000 copies/mL, the presence of lipodystrophy, and treatment with HAART for at least 18 months with no changes in the treatment regimen during the 6 months before inclusion. Exclusion criteria were the presence of opportunistic infectious disease or malignant conditions; renal disease, thyroid disease, or liver disease; body mass index greater than 30 kg/m2; fasting plasma glucose level greater than 7 mmol/L (>126.13 mg/dL), triglyceride level greater than 10 mmol/L (>884.96 mg/dL), or total cholesterol level greater than 8 mmol/L (>308.89 mg/dL); alcohol intake greater than 3 units (36 g) per day or history of alcohol abuse within the past 5 years; current thiazolidinedione therapy or known sensitivity to thiazolidinediones; clinical evidence of congestive heart failure; use of any other investigational agent within the past 4 weeks; concurrent therapy with glucocorticoids, testosterone, anabolic steroids, or growth hormones; use of any agent that could potentially interfere or interact with rosiglitazone; or history of nonadherence to medical regimens. We also excluded patients whom we considered to be potentially unreliable and those who had any concomitant condition that, in our opinion, could interfere with the interpretation of efficacy and safety data gathered in our trial. We defined the presence of HIV lipodystrophy as self-reported symptoms of loss of subcutaneous fat (face, arms, legs, and buttocks) with or without increased abdominal girth or development of a buffalo hump. An investigator confirmed these findings before enrollment. Clinical criteria for HIV lipodystrophy are controversial, but at the start of the study, no uniformly approved objective criteria were available for diagnosing lipodystrophy (28). We used subjective criteria for lipodystrophy that have been used previously (17, 19).
Study Design
Most recruited patients were consecutively seen patients with suspected lipodystrophy. At inclusion, we obtained fasting blood specimens and performed anthropometric measurements. We estimated total body fat mass by using bioimpedance analysis (RJL Systems, Clinton Township, Michigan). we randomly assigned participants in blocks of 4 to receive rosiglitazone (8 mg/d) or metformin (2 g/d) for 26 weeks by using a computer-generated list. The investigators and patients were not blinded to drug assignment. An independent pharmacist ensured allocation concealment. We requested that participants not change their regular diet, physical exercise, and smoking habit during the study. At the end of the period, we performed the same measurements. We also evaluated patients' self-reported body fat distribution and physicians' impressions of body fat distribution by open-ended questionnaire. Patients visited the hospital after 2 months and 4 months of treatment for safety evaluation, which included an open-ended questionnaire, physical examination, and blood sampling. During these visits, we evaluated adherence to study medication by open-ended questionnaire, although we did not perform pill counting. The participants also underwent a standardized oral fat-loading test before and after treatment with the study drugs. The effects of rosiglitazone and metformin on postprandial lipid and fatty acid metabolism will be published separately. The local research ethics committee of the University Medical Center approved the study protocol, and all participants gave written informed consent.
Oral Glucose Tolerance Test
Patients visited the hospital after an overnight fast. After insertion of a cannula in the forearm for venous blood sampling, patients rested for 30 minutes before administration of the glucose load (75 g). We obtained peripheral blood samples in sodium EDTA (68.44 mmol/L) before (t = 0) and at regular 15-minute intervals up to 2 hours after glucose ingestion. We kept all EDTA blood samples on ice and centrifuged them immediately for 15 minutes at 800 g at 4 °C and stored them at -80 °C until assayed.
Cross-Sectional Computed Tomography
We performed single-slice, cross-sectional computed tomography at the L4-L5 level, as described previously (29), to assess distribution of subcutaneous and visceral abdominal fat. Briefly, we obtained a lateral scout image to identify the level of the L4 pedicle, which served as the landmark for the 1-cm single-slice image. Scan variables were 144-cm table height, 80 kV, 70 mA, 2 seconds, 1-cm slice thickness, and a 48-cm field of view. On the computed tomography image, we outlined the border of the intra-abdominal cavity and quantified total and visceral abdominal fat areas by selecting an attenuation range of -250 to -50 Hounsfield units. We calculated subcutaneous abdominal fat as the difference between total and visceral abdominal fat. An independent radiologist who was unaware of the assignment status of the patients read and analyzed the computed tomography images.
Endothelial Function
We used ultrasonography to measure nitric oxide-dependent, flow-mediated vasodilation as percentage diameter change in the brachial artery after reactive hyperemia (30). We measured this at the elbow of the patients' right arms by using a Wall Track System (Scanner 200, Pie Medical, Maastricht, the Netherlands), which consists of an ultrasound imager with a 10-MHz linear array transducer connected to a data acquisition system and a personal computer. We averaged 3 measurements to calculate a baseline diameter. By inflation of a blood pressure cuff for 5 minutes at a pressure of 200 mm Hg, ischemia was applied to the forearm distal to the location of the transducer. Ultrasonography continued for 5 minutes after cuff release, with measurements at 30-second intervals. We measured the widest lumen diameter as a measure for maximal vasodilation. We used nitroglycerin (400 mg) to determine endothelium-independent vasodilation. The same technician performed all measurements with patients supine in a quiet, temperature-controlled (21 °C) environment after at least 15 minutes of rest. The operator was unaware of the assignment status of the patients. We measured endothelial function before and 2 hours after glucose ingestion.
Laboratory Measures
We measured levels of total and high-density lipoprotein cholesterol, triglycerides, glucose, apolipoprotein B, creatinine, and serum aspartate and alanine aminotransferases by using standard laboratory procedures, as described previously in detail (31). Low-density lipoprotein cholesterol was isolated by ultracentrifugation (31). We measured free fatty acids levels by an enzymatic colorimetric method (Wako Chemicals GmbH, Neuss, Germany) (31). For free fatty acid level measurement, we added a lipase inhibitor to the plasma to block ex vivo lipolysis. We measured insulin level by using enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden). We measured C-reactive protein (CRP) levels by using a high-sensitivity method (Quantex hs-CRP kit, Biokit, Barcelona, Spain) (32). We measured adiponectin levels by using ELISA (R&D Systems, Minneapolis, Minnesota). We determined CD4 cell counts by flow cytometry and HIV viral load by ultrasensitive assay.
Statistical Analysis
We tested assumptions of normality by Kolmogorov-Smirnov tests and by review of plots. Data are presented as means and SDs for variables with symmetrical distribution and as medians and interquartile ranges for variables with skewed distributions. We obtained exact 95% CIs for median differences by considering the full distribution of between-treatment differences (33). During the oral glucose tolerance test, we calculated total integrated areas under the curves (AUCs) by the trapezoidal rule and by using GraphPad Prism, version 4.0 (GraphPad Software, San Diego, California). Primary outcome measures were insulin AUC and subcutaneous and visceral abdominal fat. The secondary outcome measure was flow-mediated vasodilation. The primary analysis was to compare treatment effects between groups. For variables with symmetrical distribution, we used t-tests on changes from baseline. For variables with skewed distributions, we compared between-treatment changes from baseline with Mann-Whitney tests. The secondary analysis was to compare changes within each group with paired t-tests or Mann-Whitney tests, as appropriate. We calculated bivariate correlations between the changes in body fat distribution upon treatment and other variables by using Spearman correlation coefficients. We determined that a sample size of 15 patients per group was necessary to detect a 25% reduction in insulin AUC, with 80% power and an {alpha} level of 0.05. Since a small dropout rate may be expected, we aimed to include 20 patients in each group. We performed calculations with SPSS/PC+ software, version 11.5 (SPSS Inc., Chicago, Illinois). Statistical significance was taken at the 5% level.
Role of the Funding Source
GlaxoSmithKline funded the study. The funding source had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
|
|
| |
| |
|
|
|