| |
Once Daily HAART vs Twice Daily: patient survey
|
| |
| |
....Patients report improved convenience & flexibility by switching to fixed dose combination abacavir/3Tc from taking abacavir/3TC twice daily. Adherence was good for both groups, but adherence was greater after switching.....
Reported by Jules Levin
GSK conducted a patient survey study comparing once vs twice daily abacavir/3TC, the Fixed Dose Regimen, to abacavir+3TC twice daily. There was a time when there were doubts that once a day therapy, so I thought it would be interesting to read this patient survey. This poster was reported at IAS-Rio.
Patients taking abacavir/3TC bid were switched to the Fixed Dose Combination of 1 pill once a day of abacavir/3TC.
The study authors concluded:
Relative to ABC+3TC BID, patients treated with ABC/3TC FDC QD reported increased satisfaction with treatment convenience and flexibility.
Patient satisfaction was determined, in part, by dosing symmetry. Compared with patients on a mixed regimen, those on a pure QD regimen showed greater improvements in overall satisfaction and in aspects of satisfaction related to convenience.
Satisfaction with treatment convenience and flexibility were enhanced by switching patients from ABC+3TC BID to ABC/3TC FDC QD.
Even patients who are virologically controlled on ABC+3TC BID may benefit from the greater convenience of the fixed dose combination formulation.
Median adherence, measured by pill count, was 93% in both groups; however, the proportion of patients achieving at least 95% adherence was greater in the ABC/3TC QD group (39%) than in the ABC+3TC BID group (31%).
Patient Satisfaction Results:
Mean HIVTSQs summary scores at baseline indicated a high level of
satisfaction in both treatment groups (ABC+3TC BID, 90; ABC/3TC QD, 91),
difference: p=0.49.
The status version (HIVTSQs) was administered at baseline to assess patient
satisfaction with the HIV treatment regimen (s)he was receiving at study entry.
HIVTSQs items were rated on a 7-point scale (0-6); higher scores indicate
greater satisfaction. A total score was calculated by summing all ten items
(0-60) and transforming the summary score to a 0-100 scale.
The change version (HIVTSQc) was administered at all post-baseline visits to
evaluate how a patient rated his/her on-study treatment compared with the
treatment (s)he was receiving at study entry. HIVTSQc items were rated on a
7-point scale (-3 to +3); positive scores indicate greater satisfaction with study
treatment while negative scores indicate lesser satisfaction with study
treatment compared to the previous regimen.
"Patient Satisfaction with Abacavir (ABC)-Lamivudine (3TC)
Fixed Dose Combination (FDC) Tablet Once Daily (QD)
Compared with ABC and 3TC Twice Daily (BID) in HIV-1
Infected Patients (ESS30008)"
M. Watson1, C. Hill-Zabala1, N. Sosa2, E. DeJesus3, A. Florance1
1GlaxoSmithKline, Research Triangle Park, North Carolina; 2Social Security Hospital, Panama City, Panama;
3IDC Research Initiative, Altamonte Springs, Florida
At Week 48, there were trends towards greater improvement in satisfaction
with treatment convenience (p=0.041) and treatment flexibility (p=0.074)
among patients who received ABC/3TC QD.
Figure 2. Proportion of Patients with Improved Satisfaction
(scoring "+2" or "+3") on the HIVTSQc at Week 48
This graph shows patient survey found improved regimen convenience & flexibility for FDC abacavir/3TC compared to twice daily abacavir/3TC.
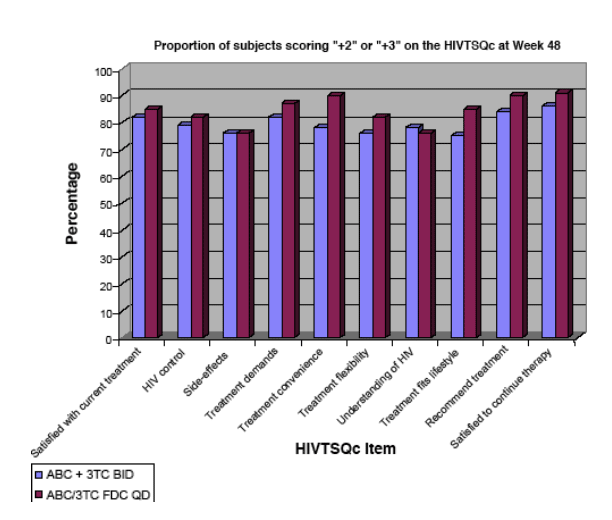
Table 1. Mean HIVTSQc Scores at Week 48
This chart shows better satisfaction scores for FDC compared to twice daily regimen.
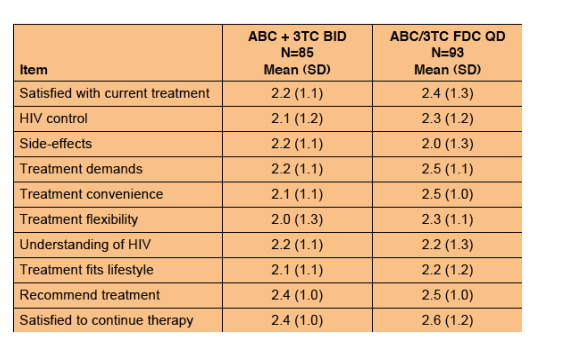
Figure 3. Proportion of Patients with Improved Satisfaction
(scoring "+2" or "+3") on the HIVTSQc at Week 48 by
Symmetry of Dosing
This chart shows patients preferred qd & bid to a mixed dose regimen which included both a qd & bid drug.
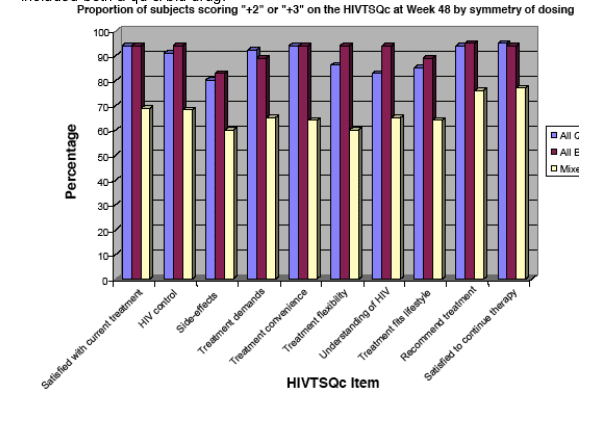
The overall effect of dosing symmetry was statistically significant for 6 HIVTSQc items (satisfied with current treatment, treatment demands, treatment convenience, treatment flexibility, treatment fits lifestyle and satisfied to continue treatment; p<0.05 for each). These overall effects were driven by the comparison between the pure QD and mixed regimens, with patients on a pure QD regimen showing significantly greater improvements in satisfaction compared with patients whose antiretroviral regimen included both a BID and a QD component.
As part of a study designed to evaluate the efficacy and safety of the ABC/3TC FDC QD compared to treatment with the separate components BID, patient satisfaction was measured.
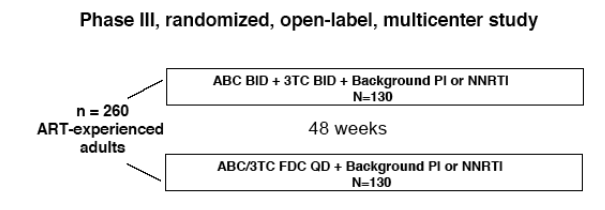
The primary clinical endpoint was the proportion of patients who did not fail
virologically or discontinue study drug (responders). Virologic failure was defined
as confirmed plasma HIV-1 RNA ≥ 1265 copies/mL (i.e., 0.5 log10 copies/mL
over 400 copies/mL). Week 48 clinical (safety and efficacy) and adherence data
have been presented separately1.
Patient satisfaction was measured using an updated version of the HIV Treatment Satisfaction Questionnaire (HIVTSQ)2. This self-completed questionnaire now contains 10 questions that assess general satisfaction with,
as well as the ease of taking, an HIV regimen. Two versions of the HIVTSQ were
used:
--The status version (HIVTSQs) was administered at baseline to assess patient
satisfaction with the HIV treatment regimen (s)he was receiving at study entry.
HIVTSQs items were rated on a 7-point scale (0-6); higher scores indicate
greater satisfaction. A total score was calculated by summing all ten items
(0-60) and transforming the summary score to a 0-100 scale.
--The change version (HIVTSQc) was administered at all post-baseline visits to
evaluate how a patient rated his/her on-study treatment compared with the
treatment (s)he was receiving at study entry. HIVTSQc items were rated on a
7-point scale (-3 to +3); positive scores indicate greater satisfaction with study
treatment while negative scores indicate lesser satisfaction with study
treatment compared to the previous regimen.
Results
260 patients were enrolled from the United States, Costa Rica, Panama, and
Puerto Rico. Because a Latin American (LA) Spanish translation of the HIVTSQ
was not available however, most LA patients were unable to complete the
questionnaire. Of the enrolled patients, 182 (95 ABC/3TC QD and 87 ABC+3TC
BID) completed at least one HIVTSQ questionnaire and were included in this
analysis. The HIVTSQ population was primarily male (84%) and racially diverse
with 53% white, 34% black, and 12% Hispanic patients.
Clinical Results:
A high proportion of patients in both treatment groups were responders
(non virologic failures, ABC+3TC BID, 93%; ABC/3TC QD, 95%) at 48 weeks.
These data established the noninferiority of ABC/3TC administered QD
compared with ABC+3TC administered as separate components BID.
Both regimens were very well tolerated. The incidence of grade 2-4 AEs was
low and comparable between treatment groups. No patient withdrew from the
study due to a treatment-related AE. No cases of ABC hypersensitivity reaction
were reported in this ABC-experienced population.
Median adherence, measured by pill count, was 93% in both groups; however,
the proportion of patients achieving at least 95% adherence was greater in the ABC/3TC QD group (39%) than in the ABC+3TC BID group (31%).
|
|
| |
| |
|
|
|