| |
PA-457 Panacos Drug May Combat Resistant AIDS Strains
|
| |
| |
By MARILYN CHASE
THE WALL STREET JOURNAL
August 22, 2005
Panacos Pharmaceuticals Inc. said a study showed its experimental AIDS drug PA-457 reduced blood levels of the human immunodeficiency virus by 90% in a small study of HIV-infected volunteers.
PA-457 is the first member of a new class of antiviral drugs that block maturation of HIV, the virus that causes AIDS, by causing the release of virus particles incapable of spreading the infection in the body. Although the study was small and limited in duration, scientists are encouraged because the drug is active against strains that have developed resistance to other drugs.
Shares of Panacos surged on the news, rising $3.25, or 46%, to $10.30, in 4 p.m. composite trading on the Nasdaq Stock Market.
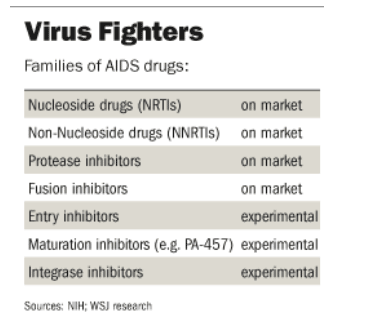
Doctors are in a constant race to devise regimens that stay a step ahead of resistance spawned by the constantly mutating virus. Carl Dieffenbach, director of basic science in the U.S. Division of AIDS at the National Institute of Allergy and Infectious Diseases, said the prospect of a new family of antivirals is good news. "The larger armamentarium will make a big difference" to those on AIDS treatment, he said.
In the study, 32 people with HIV/AIDS received a once-daily pill of PA-457 or an inactive placebo pill, for 10 days. Blood levels of HIV fell a median 90% in people who took the highest dose of 200 milligrams. Main side effects included nausea and headache. One volunteer with a history of high blood pressure suffered a stroke, which Panacos said was unrelated to the study treatment.
Although the data are preliminary, Graham Allaway, Panacos's chief operating officer, said they potentially point to "an extremely potent drug." The company hopes to present compete data from the study at the Interscience Conference on Antimicrobial Agents and Chemotherapy in New Orleans in September.
Panacos, Watertown, Mass., said it hopes to launch pivotal Phase 3 efficacy trials by early 2007 and file for market approval by 2008. If successfully launched, the drug, derived from the bark of the European plane tree, could fetch $500 million to $1 billion a year in sales, Panacos projects.
|
|
| |
| |
|
|
|