| |
Response to hepatitis A vaccine in HIV patients in the HAART era: CD4 count at time of vaccination determines response to vaccine
|
| |
| |
AIDS: Volume 19(15) 14 October 2005 p 1702-1704
Rimland, David; Guest, Jodie L
VA Medical Center and Emory University School of Medicine, Atlanta, GA, USA.
".....We found that the CD4 cell count at the time of vaccination is the critical determinant of response to hepatitis A vaccine. Regardless of the nadir CD4 cell count, our data suggest that patients will respond to vaccine after HAART-associated immunological reconstitution.... We would suggest that patients receiving antiretroviral therapy should wait to receive vaccines, in particular hepatitis A vaccine, until immunological reconstitution, indicated by CD4 cell increases to more than 200 cells/ml, has been achieved..... The determination of anti-HAV IgG antibody should be considered in all HIV patients receiving hepatitis A vaccine. A lack of detectable IgG antibody would suggest repeating vaccination, especially if current CD4 cell counts exceed 200 cells/ml....." see table below, response rates appear greater when CD4s were >500 compared to 250-500.
Abstract
We evaluated the development of hepatitis A antibody after vaccination in a large cohort of patients studied in a clinical setting after the introduction of HAART. Overall, 130 of 214 vaccinated individuals developed hepatitis A antibody. In a multivariate analysis, only the CD4 cell count at the time of vaccination was associated with an absence of response. The lack of association with the nadir CD4 cell count suggests that patients will respond to vaccine after immunological reconstitution in response to HAART.
Hepatitis A virus (HAV) is a common infection in HIV-infected patients and in patients at risk of HIV infection, including men who have sex with men (MSM) and injection drug users [1-6]. Hepatitis A vaccine has been available since 1995 and is recommended for HIV patients, especially MSM, injection drug users and individuals with chronic liver disease [7,8]. A recent study suggested that a minority of eligible patients receive hepatitis A vaccine [9]. Several studies have concluded that HIV patients may have a poorer response to hepatitis A vaccine, but these studies were carried out before the HAART era [10,11]. More recent studies evaluating the response to hepatitis A vaccine were carried out, but the effect of HAART was not evaluated [12-14]. Factors determining a poor response to vaccine have not been well defined in the HAART era.
The HIV Program has followed all HIV-infected patients seen at the Atlanta VA Medical Center since 1982. We have prospectively collected demographic, clinical and laboratory data on all patients as part of the HIV Atlanta VA Cohort Study (HAVACS). Since 1992, 1319 patients have been tested for hepatitis A antibody (anti-HAV IgG). Positive anti-HAV was found in 583 patients before vaccination, documenting the high prevalence of hepatitis A in these patients. Of those testing anti-HAV negative, 659 were vaccinated with two doses of inactivated hepatitis A vaccine (HAVRIX) between 1996 and 2003.
All anti-HAV testing was done as part of the routine clinical care for these patients. A decision to measure the post-vaccination response was made by the individual providers.
Of the vaccinated patients, 214 were subsequently tested for anti-HAV IgG to evaluate the response using the ETI-AB-HAVK PLUS assay (DiaSorin SpA, Saluggia, Italy). Although the total HAV antibody test has not previously been recommended post-vaccination because of a high limit of detection of 100 mIU/ml using previous methods, this assay has been shown to have a lower limit of only 20 mIU/ml, similar to the level needed for protection from natural disease [8].
We determined the effect of demographic, clinical and laboratory factors on the response to vaccine by univariate and multivariate logistic regression analysis. Statistical significance was determined on the basis of an alpha level of 0.05.
Overall, 130 out of 214 vaccinated individuals (60.7%) developed a positive anti-HAV IgG antibody. Response to vaccination was directly related to the CD4 cell count at vaccination (Fig. 1). The higher the CD4 cell count, the higher the likelihood of detectable anti-HAV IgG. By univariate analysis, a lower CD4 cell count at the time of vaccination, a lower nadir CD4 cell count and a higher HIV viral load were associated with a lack of response to vaccine. Age, race, HIV risk factor, the use of HAART and the presence of hepatitis C virus infection were not associated with the response. In a multivariate analysis, only the CD4 cell count at the time of vaccination was associated with an absence of response (P < 0.0001). A dose response was seen with a higher CD4 cell count at vaccination being associated with higher response rates to vaccination independent of the nadir CD4 cell count and viral load level. Patients with a CD4 cell count less than 200 cells/ml were 16 times more likely to be non-responders; a CD4 cell count of 200-500 cells/ml was associated with a 2.5 increased risk of non-response.
Fig. 1. Response to hepatitis A vaccine by CD4 cell count at vaccination.
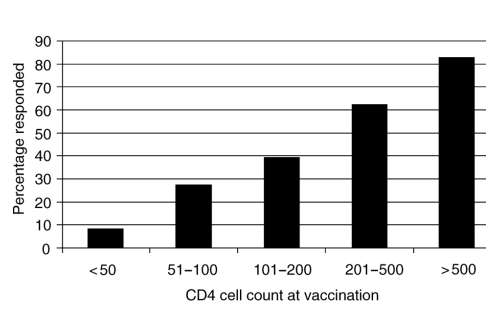
We found that the CD4 cell count at the time of vaccination is the critical determinant of response to hepatitis A vaccine. Regardless of the nadir CD4 cell count, our data suggest that patients will respond to vaccine after HAART-associated immunological reconstitution. Early reports suggested that HIV-infected patients did not respond well to hepatitis A vaccine. A study carried out in patients with hemophilia demonstrated that seroconversion rates and antibody titers were significantly lower than in non-HIV-infected patients [15]. Two studies performed in MSM in the pre-HAART era suggested that HIV infection was associated with a lower rate of seroconversion than in non-infected men [10,11]. Three additional studies were carried out in patients receiving HAART, but few factors were evaluated with respect to the response rate. Valdez et al. [12] evaluated 31 patients receiving a ritonavir-containing regimen and found that 11 out of 15 patients (73%) responded to vaccine. Kemper et al. [13] studied 133 patients in a prospective, placebo-controlled trial during the HAART era, but they did not collect information on treatment. They found that patients with a CD4 cell count greater than 200 cells/ml had a 68% seroconversion rate compared with only 9% in patients with CD4 cell counts less than 200 cells/ml. Wallace et al. [14] studied 90 patients in the navy; 77% were on protease inhibitors. They found that 100% of patients with a CD4 cell count greater than 300 cells/ml seroconverted, compared with only 87% of patients with a CD4 cell count less than 300 cells/ml [14].
Two recent unpublished studies add further data. Weissman et al. [16] found that only 50% of HIV-positive patients responded to hepatitis A vaccine. Responders had higher CD4 cell counts and were more likely to be women. Overton et al. [17] reported that only the suppression of viral replication (viral load < 1000 copies/ml) at the time of vaccination was independently associated with the response to vaccination; they found no independent association with a nadir CD4 cell count or the CD4 cell count at the time of vaccination. We have no explanation for the different results in this last study compared with our results.
It is not surprising that HIV-infected individuals do not respond as well to hepatitis A vaccine. Several vaccines, including those against influenza, tetanus, pneumococcal infection and hepatitis B, have been found to produce a decreased response in these patients [18-20]. It has previously been suggested that patients should receive vaccinations before immunological deterioration [7]. No specific recommendations have been made about patients presenting with low CD4 cell counts. We would suggest that patients receiving antiretroviral therapy should wait to receive vaccines, in particular hepatitis A vaccine, until immunological reconstitution, indicated by CD4 cell increases to more than 200 cells/ml, has been achieved.
The determination of anti-HAV IgG antibody should be considered in all HIV patients receiving hepatitis A vaccine. A lack of detectable IgG antibody would suggest repeating vaccination, especially if current CD4 cell counts exceed 200 cells/ml. The CD4 cell count at the time of vaccination is the critical determinant of response to HAV vaccine. The absence of an association with a nadir CD4 cell count suggests that patients will respond to vaccine after immunological reconstitution in response to HAART.
|
|
| |
| |
|
|
|