| |
Efavirenz detectable in plasma 8 weeks after stopping therapy and subsequent development of non-nucleoside reverse transcriptase inhibitor-associated resistance
|
| |
| |
AIDS: Volume 19(15) 14 October 2005 p 1716-1717
Sadiq, S Tariqa; Fredericks, Salimb; Khoo, Saye Hc; Rice, Phillipd; Holt, David Wb
aCentre for Infection Division Cellular and Molecular Medicine
bAnalytical Unit, St George's, University of London
cDepartment of Pharmacology, University of Liverpool
dDepartment of Virology, St George's Healthcare NHS Trust, UK.
Below is a letter to the journal AIDS explaining the experience of a patient who abruptly stopped taking efevirenz & developed EFV drug resistance. This letter discusses a case of slow elimination of EFV in a patient after stopping therapy. It took 2 months for EFV levels to become undetectable after stopping therapy. This is a setup for developing drug resistance. In addition, the patient previously had stopped EFV, which may have also contributed to developing EFV resistance when stopping later on. It is known that HIV drugs are eliminated from the body at varying rates. EFV & all NRTIs are eliminated more slowly. As well, drug resistance can develop more easily for certain drugs because only 1 resistance mutation is needed to develop high level resistance which can develop quickly. We know resistance to EFV, NVP, & 3TC can occur with the development of 1 mutation & this can occur quickly. Resistance to protease inihibitors in general requires several mutations & resistance to a PI takes longer & is more difficult to develop. The point is that although EFV has received attention regarding this concern the development of drug resistance after abruptly stopping HAART can occur with other HIV drugs. It is recommended that if a patient wants to stop therapy they should speak to their doctor. Stopping HAART needs to be done in an planned & intelligent way. It is recommended to start a PI such as Kaletra and add it to the regimen several weeks before stopping a NNRTI. However, the authors question the recommendation & suggest it is difficult to know for sure how an individual will eliminate a drug. Thus, treatment interruption of any HAART regimen is associated with risk.
AUTHORS SAY:
Both US and UK HIV treatment guidelines acknowledge the difficulty of knowing the exact duration of antiretroviral therapy cover required when stopping NNRTI. Clearly, a few days or even weeks may not be enough in some patients. Until further research from treatment interruption trials has clarified the issue, it may be clinically inappropriate to guess the period of drug cover required, particularly in those from 'at-risk' groups. Monitoring the decay of plasma drug concentrations (ideally with as rapid a turnaround as possible) may be a wiser approach to manage NNRTI treatment interruptions. In future, there may be a place for near-patient drug testing and pharmacogenetics to guide such decisions.
ARTICLE TEXT
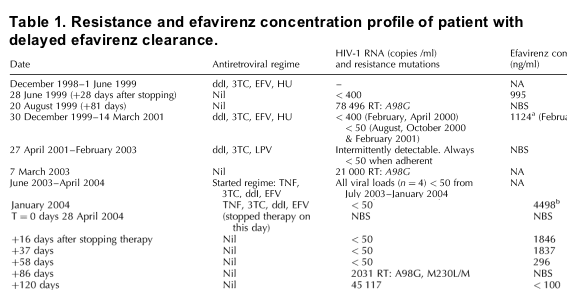
A 40-year-old black African HIV-1-positive woman maintained an undetectable blood plasma HIV-1-RNA concentration (< 50 copies/ml) for 10 months while receiving efavirenz (600 mg a day), tenofovir (245 mg a day), lamivudine (300 mg a day) and didanosine (400 mg a day). When her supply of drugs ran out she stopped therapy abruptly, because she thought she was pregnant, but first informed her physician of her decision 4 weeks later. The patient elected to remain off antiretroviral therapy, but plasma HIV-1-RNA concentrations remained undetectable repeatedly up to 8 weeks after stopping therapy, with virus first detectable at 12 weeks. The patient denied taking antiretroviral therapy during this time and an examination of pharmacy scripts confirmed she had had no new or existing supplies from the clinic. Population-based sequencing of the reverse transcriptase gene of the virus from the 12-week sample showed the presence of a mutant/wild-type mix, M230L/M, associated with non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance. Measurement of the efavirenz concentration on available archived blood plasma samples after she had stopped therapy showed detectable concentrations at 2 (1846 ng/ml), 5 (1837 ng/ml) and 8 (296 ng/ml) weeks after stopping therapy, and undetectable (< 100 ng/ml) concentrations at 17 weeks. The efavirenz concentration from an available archived plasma sample from when she was taking the drug (6 months earlier), and for which the timing of sample and dose were known, was 5309 ng/ml, giving a projected trough concentration of 4498 ng/ml. The patient was not taking any other treatments (including anti-tuberculous medication) at the time of stopping therapy, and denied ever taking grapefruit or cranberry juice.
The patient had also stopped an efavirenz-containing regimen 5 years previously. The efavirenz concentration measured on an available archived plasma sample, taken 4 weeks after stopping this regimen (when the HIV-1 viral load was less than 400 copies/ml), was also significant (995 ng/ml). Genotypic sequencing performed retrospectively on an archived blood plasma sample taken 3 months after stopping this regimen, and on another archived sample taken during a treatment break from a subsequent protease inhibitor-containing regime, did not demonstrate any significant NNRTI-associated mutations.
The patient consented to having her genetic polymorphisms investigated. The patient's DNA was extracted from whole blood and genotyped for the P450 iso-enzymes CYP3A5, CYP2B6 [1], CYP3A4 * 1B and the C3435T mutation of the MDR-1 gene. The following genotypes were demonstrated: CYP2B6: exon 1, C64T, CC; exon 4, G516T, GT; exon 5, C777A, CC; exon 5, A785G, GG; exon 9, C1459T, CC. CYP3A4: A392G, AA. CYP3A5: A6986G, *1/*3, (AG). MDR-1: exon 26, C3435, CT.
Previous work has suggested that the maximum time for efavirenz excretion may be 2 weeks (t1/2 23-36 h) [2,3], but in black Africans it may be as long as 3 weeks, and perhaps longer still for those on P450 iso-enzyme inhibitory drugs. Our patient, receiving no such therapy, took at least 8 weeks to excrete efavirenz fully, with the apparent evolution of NNRTI resistance as the virus became detectable. Interestingly, efavirenz concentrations at the 2 and 5-week timepoints were very similar and did not follow an expected decay pattern. Repeat checking of labelling revealed no errors, and the samples were retested giving similar results. Furthermore, the delayed clearance of efavirenz was confirmed by demonstrating significant drug concentrations 4 weeks after stopping efavirenz therapy on a previous occasion (Table 1).
ED NOTE from Jules Levin:
In the table below you can see the patient previous to her stopping EFV due to pregnancy April 2004 had stopped EFV & had low EFV levels. These prior experiences may have played a role in EFV resistance development when she subsequently stopped EFV. In the last 7-8 lines of the table you can see EFV concentrations were 4498 ng/ml in Jan 2004 when the patient was on a regimen of TNF/3TC/ddI/EFV. After stopping therapy EFV concentrations slowly declined over the next 58 days (2 months). Such a slow elimination of EFV allows HIV to replicate in the presence of drug which is a setup for drug resistance. Although the study looks at EFV & EFV has been previously studied & the subject of this type of research, this type of setup for resistance can occur with other drugs. Nevirapine & 3TC are also drugs for which resistance can occur easily if HAART is stopped abruptly. Numerous studies have shown that upon treatment interruption drug resistance can develop in a portion of patients. Different drugs are eliminated from the body at different rates and this can contribute to drug resistance developing. If a patient had any pre-existing drug resistance perhaps from missing drugs for a couple of days two years earlier this can contribute to drug resistance emergingf upon abrupt stopping of HAART later on.
Single nucleotide polymorphisms (SNP) in the CYP2B6 (G516T) and CYP3A4 (A392G) genes have been shown to be associated with increased drug concentrations of efavirenz [4]. This is particularly true for G516T in black Africans in whom this SNP is more common compared with Caucasians. This patient was wild type for CYP3A4. In CYP2B6, she carried G516T heterozygously, A785G homozygously but was wild type for all other known CYP2B6 SNP. This particular combination of SNP, the *4/*6 variant is rare in Caucasians (< 1%) but its prevalence in black Africans and its in-vivo effects on efavirenz are unknown [1].
Both US and UK HIV treatment guidelines acknowledge the difficulty of knowing the exact duration of antiretroviral therapy cover required when stopping NNRTI. Clearly, a few days or even weeks may not be enough in some patients. Until further research from treatment interruption trials has clarified the issue, it may be clinically inappropriate to guess the period of drug cover required, particularly in those from 'at-risk' groups. Monitoring the decay of plasma drug concentrations (ideally with as rapid a turnaround as possible) may be a wiser approach to manage NNRTI treatment interruptions. In future, there may be a place for near-patient drug testing and pharmacogenetics to guide such decisions.
References
1. Lang T, Klein K, Fischer J, Nussler AK, Neuhaus, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 2001; 11:399-415.
2. Molina JM, Peytavin G, Perusat S, Lascoux-Combes C, Sereni D, Rozenbaum W, Chene G. Pharmacokinetics of emtricitabine, didanosine and efavirenz administered once-daily for the treatment of HIV-infected adults (pharmacokinetic substudy of the ANRS 091 trial). HIV Med 2004; 5:99-104
3. Taylor S, Allen S, Fidler S, White D, Gibbons S, Fox J, et al. Stop Study: after discontinuation of efavirenz, plasma concentrations may persist for 2 weeks or longer. In 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, 8-11 February 2004 [Abstract 131].
4. Haas DW, Ribaudo JH, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18:2391-2400.
|
|
| |
| |
|
|
|