| |
Transient Viremia in HIV-Infected Patients and Use of Plasma Preparation Tubes
|
| |
| |
Clinical Infectious Diseases Dec 1, 2005;41:000
Valentina Stosor,1 Frank J. Palella, Jr.,1 Baiba Berzins,1 Michele Till,1 Angel Leake,1 Joan S. Chmiel,2 and Robert L. Murphy1
1Division of Infectious Diseases and 2Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois
Using plasma preparation tubes for the collection and storage of plasma resulted in factitious, low-level human immunodeficiency virus type 1 (HIV-1) viremia among patients receiving highly active antiretroviral therapy who incurred unnecessary additional clinic visits, laboratory testing, and medication changes. We caution clinicians against the routine use of plasma preparation tubes for collection of blood samples for HIV-1 level 1 quantification.
.....We observed a high incidence (66.1%) of transient viremia during the period of PPT use. Transient viremia was significantly more likely to occur during use of PPTs than after use of EDTA tubes had resumed (P < .0001).... More patients experienced viremia during the PPT period than during the subsequent EDTA tube period (39 patients [69.6%] vs. 3 patients [5.4%]; P < .0001)....
.... PPTs were used in a major, randomized, comparative trial involving antiretroviral therapynaive persons who received treatment with either efavirenz or atazanavir plus zidovudine and lamivudine. Because viral suppression was unexpectedly low in both treatment arms (32% of atazanavir recipients and 37% of efavirenz recipients), investigators performed additional laboratory assessments with paired stored plasma specimens simultaneously collected using PPTs and EDTA tubes. Ninety-five percent of EDTA-collected specimens analyzed with the HIV-1 Monitor assay (Amplicor) versus 84% of the PPT-prepared specimens analyzed with the Standard assay demonstrated viral loads of <400 copies/mL, and 89% of EDTA-collected specimens versus 55% of PPT-collected specimens demonstrated viral loads of <50 copies/mL by the UltraSensitive method.....
Table 1. Subsequent virologic outcomes measured in 56 virally suppressed HAART recipients during a period of Vacutainer plasma preparation tube (PPT; Becton-Dickinson Vacutainer Systems) use and after resumption of standard ethylenediaminetetraacetic acid (EDTA) tube use.
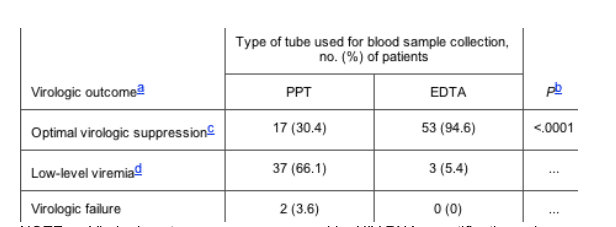
NOTE. Virologic outcomes were measured by HIV RNA quantification using the Roche Amplicor Monitor Assay, version 1.5, during the period of PPT use and, subsequently, during the period of EDTA tube use. At least 1 viral load measurement was obtained from all patients during each time period.
a Definitions of virological outcomes are as follows: optimal virologic suppression, an HIV load of <50 copies/mL; low-level viremia, an HIV load of 502000 copies/mL; and virological failure, an HIV load of >2000 copies/mL.
b Low-level viremia and virologic failure categories were collapsed for purposes of statistical analysis. Two-sided P value compares PPT vs. EDTA virologic outcomes, using McNemar's test.
c Optimal viral suppression was maintained in 17 patients throughout both PPT and EDTA tube use periods.
d Persistent low-level viremia was detected in three patients during both PPT and EDTA tube use periods.
Plasma preparation tubes (PPTs) are a single-tube system used to collect and process blood samples for HIV-1 quantification [1]. Compared with standard collection tubes, PPTs are used with the same anticoagulant, K2 EDTA, but they contain a polyester gel barrier for separating blood cells from the acellular component. Use of PPTs results in a plasma yield containing more platelets and cellular debris than does plasma prepared from EDTA-processed specimens. Despite this, previous studies have demonstrated the suitability of PPTs for use in quantification of plasma HIV-1 level [2, 3].
During January-August 2003, we used Vacutainer PPTs (Becton-Dickinson Vacutainer Systems) in lieu of standard EDTA tubes to collect blood samples for HIV-1 quantification. We subsequently observed an increase in the occurrence of low-level viremia among HAART recipients with previously suppressed virus. We undertook this analysis to identify potential clinical factors other than use of PPTs associated with low-level viremia. We also examined the clinical impact of PPT-based assays indicating transient viremia.
Materials and methods.
The Centers for Disease Control and Preventionsponsored Northwestern HIV Outpatient Study (HOPS) is a prospective observational cohort study into which ambulatory patients are continuously recruited from the HIV Center of Northwestern Memorial Hospital (Chicago, IL). This analysis includes data from 595 patient visits, from January 2002 through February 2004. The institutional review board of Northwestern University approved this study.
The HOPS electronic database was surveyed for HIV-1infected HAART recipients who met the following criteria: 3 consecutive plasma HIV-1 RNA load measurements of <50 copies/mL during January-December 2002, when EDTA tubes were routinely used; >1 viral load measurement during January-August 2003, when PPTs were used; and >1 reported viral load measurement during SeptemberDecember 2003, when use of EDTA tubes recommenced.
Transient viremia was defined as a viral load of 502000 copies/mL after 3 consecutive viral load measurements of <50 copies/mL during the previous year and followed by at least 1 subsequent viral load measurement of <50 copies/mL. Virological failure was defined as a viral load of >2000 copies/mL after 3 consecutive viral load measurements of <50 copies/mL during the previous year.
From January 2002 through December 2002, samples for viral load testing were collected in Vacutainer EDTA tubes (Becton-Dickinson Vacutainer Systems). From January 2003 through August 2003, blood samples were collected and processed in Vacutainer PPTs. Beginning in September 2003, the laboratory ceased use of PPTs and resumed use of EDTA tubes. All tubes were centrifuged and processed in accordance with the manufacturer's instructions. Plasma samples were removed from EDTA tubes before storage, whereas plasma samples in PPTs were stored in situ at -70 C within 1 h after processing, until the time of testing.
Viral load testing was performed using the Roche Amplicor HIV-1 Monitor test UltraSensitive method (Roche Molecular Systems). The HIV-1 Monitor test, version 1.0, was used from January 2002 until 2 November 2002, when the laboratory switched to version 1.5. All testing was performed as part of routine patient care.
Data were abstracted from Northwestern's HOPS database and by means of additional review of outpatient medical records covering 3 distinct time periods: the 12-month period before detection of viremia, the period spanning the duration of transient viremia, and the 6-month period after transient viremia was detected. Patient data included the following: demographic characteristics, insurance status, number and nature of outpatient clinic visits, pharmacy counseling sessions, clinical trial participation, details regarding receipt of antiretroviral therapy, patient-reported therapy adherence, and receipt of vaccines within 6 weeks before viral load testing. We abstracted data regarding the frequency and timing of viral load tests, CD4 cell measurements, resistance assays, and antiretroviral therapy pharmacokinetic studies.
Data were coded and entered into a standard electronic spreadsheet. Low-level viremia and virologic failure categories were collapsed for the purpose of analysis. Within-patient differences in virologic outcome for the different tube-type use periods were evaluated by a 2-sample test (McNemar's test) for binomial proportions for matched-paired data.
Results.
Fifty-six HAART recipients had viral loads that were undetectable at 3 consecutive measurements during the year before PPT use. Table 1 summarizes virologic outcomes for these patients, stratified by type of tube used during the 8-month period of PPT use and during the subsequent 6-month period of EDTA tube use. More patients experienced viremia during the PPT period than during the subsequent EDTA tube period (39 patients [69.6%] vs. 3 patients [5.4%]; P < .0001). Overall, low-level viremia was detected in 37 patients (66.1%). Thirty-four patients (60.7%) experienced low-level viremia that subsequently resolved on resumption of EDTA tube use, whereas only 3 patients (5.4%) experienced low-level viremia during the period of PPT use that persisted after use of EDTA tubes resumed. Viral suppression occurred in only 17 patients (30.4%) during the period of PPT use. Two patients (3.6%) experienced virologic failure during the period of PPT use.
Among the 34 patients who experienced low-level viremia only during the period of PPT use, the median age was 47 years (range, 28-63 years), and there was a predominant number of men (85.3%). Ethnic profiles of patients closely resembled those of the entire Northwestern HOPS cohort. Eighteen (52.9%) of 34 patients were receiving protease inhibitorbased HAART, 12 patients (35.3%) were receiving nonnucleoside reverse-transcriptase inhibitorbased regimens, 3 patients (8.8%) were receiving combination protease inhibitorbased and nonnucleoside reverse-transcriptase inhibitorbased regimens, and 1 patient (2.9%) was receiving a triplenucleoside reverse-transcriptase inhibitor regimen. The median nadir CD4 cell count was 224 cells/mm3 (range, 2-824 cells/mm3). The median CD4 cell count and percentage among values obtained at clinic visits immediately preceding episodes of viremia were 447 cells/mm3 (range, 59-1405 cells/mm3) and 25% (range, 10%-41%), respectively. Six patients (17.6%) were clinical trial participants.
Among patients with PPT-associated transient viremia, the median viral load measured in 80 collected specimens was 80 copies/mL (range, 0-1194 copies/mL). Three viral load measurements that were obtained using the Standard assay (limit of quantification, 400 copies/mL) resulted in a mean of 632 copies/mL (range, 619-652 copies/mL). The mean number of viral load measurements per patient was 1.7 (range, 1-4 measurements).
Clinical events preceding or coincident with episodes of transient viremia ("blips") are summarized in table 2. Median CD4 cell counts and percentages were 422 cells/mm3 (range, 53-1369 cells/mm3) and 24% (range, 10%-41%), respectively. Four patients (11.8%) reported nonadherence to medication. Acute illnesses occurred in 7 patients (20.6%). One patient was immunized within 6 weeks before the viral blip. In summary, 12 patients (35.3%) experienced clinical events in close temporal proximity to detection of low-level viremia during the period of PPT use.
Thirteen patients (38.2%) required at least 1 unplanned additional clinic visit and underwent at least 1 repeated viral load measurement, because of transient viremia. In 2 instances, viremia was further investigated with resistance testing, prompting a medication change for 1 patient. One patient underwent pharmacokinetic studies to confirm therapeutic protease inhibitor levels. The median CD4 cell count and percentage for the postviremic period were 432 cells/mm3 (range, 5041760 cells/mm3) and 24% (10%41%), respectively.
Discussion.
For HAART recipients, periodic measurements of viral load is essential for monitoring therapeutic responses and virologic progression [4, 5] and is an established endpoint in clinical trials [6]. Standardization of specimen collection procedures and viral load assays is essential to limit variability in intrapatient and interpatient comparisons and in cross-regimen comparisons. Although previous laboratory assessments have demonstrated viral load stability in specimens that were collected, frozen, stored, and shipped in PPTs, such studies were performed using specimens with viral loads of >5000 copies/mL [2, 3].
We observed a high incidence (66.1%) of transient viremia during the period of PPT use. Transient viremia was significantly more likely to occur during use of PPTs than after use of EDTA tubes had resumed (P < .0001). Medical events temporally associated with transient viremia were too infrequent to explain observed occurrences of viremia. Although specimens simultaneously processed and stored in both EDTA tubes and PPTs were not available for intrapatient comparison, all patients had suppressed virus prior to PPT use, and most (94.6%) were virally suppressed after resumption of EDTA tube use, clearly suggesting a causative association between "breakthrough" viremia and PPT use.
PPTs were used in a major, randomized, comparative trial involving antiretroviral therapynaive persons who received treatment with either efavirenz or atazanavir plus zidovudine and lamivudine [7]. Because viral suppression was unexpectedly low in both treatment arms (32% of atazanavir recipients and 37% of efavirenz recipients), investigators performed additional laboratory assessments with paired stored plasma specimens simultaneously collected using PPTs and EDTA tubes. Ninety-five percent of EDTA-collected specimens analyzed with the HIV-1 Monitor assay (Amplicor) versus 84% of the PPT-prepared specimens analyzed with the Standard assay demonstrated viral loads of <400 copies/mL, and 89% of EDTA-collected specimens versus 55% of PPT-collected specimens demonstrated viral loads of <50 copies/mL by the UltraSensitive method.
Our observations, conducted in a real-life, outpatient setting, confirm that PPT use is associated with an unacceptably and probably erroneously high frequency of low-level viremia. This, in turn, resulted in an increase in the use of medical resources, including laboratory testing and outpatient visits. One patient underwent a presumably unnecessary change in antiretroviral therapy.
The recent release of a "white paper" [8] by the PPT manufacturer supports our findings and offers revised sampling procedures, including the removal of plasma from the tube before freezing. However, this added step eliminates the convenience and greater safety offered by this more costly product, compared with the standard EDTA tube.
Our study is limited by its retrospective nature. Prospective comparisons of collection tubes and specimen processing will result in a better standardization of these procedures. Additional investigations are needed to determine the source of HIV-1 RNA detected in PPT-collected specimens.
We conclude that, for patients with viral suppression, viral load measurements performed with specimens collected and stored in PPTs result in factitious low-level viremia and consequent increases in the use of health care resources, including unnecessary plasma sampling and patient and physician inconvenience. We caution against the use of PPTs for viral load testing in both clinical and research settings.
|
|
| |
| |
|
|
|