| |
EFV CNS Effects & Blood levels
|
| |
| |
"Prediction of Neuropsychiatric Adverse Events Associated with Long-Term Efavirenz Therapy, Using Plasma Drug Level Monitoring"
Felix Gutierrez,1 Andres Navarro,2 Sergio Padilla,1 Rosa Anton,2 Mar Masia,1 Joaquin Borras,2 and Alberto Martin-Hidalgo1
1Infectious Diseases Unit, Internal Medicine Department, and 2Clinical Pharmacy Section, Hospital General Universitario de Elche, Alicante, Spain
Clinical Infectious Diseases Dec 1, 2005;41:000
....our study provides indirect evidence for a higher risk of CNS-related adverse events among patients with higher efavirenz plasma concentrations who receive long-term antiretroviral therapy with efavirenz-containing regimens. Monitoring of plasma levels should allow patients with higher efavirenz plasma levels who are, consequently, at increased risk for neuropsychiatric adverse events to be detected.....
ABSTRACT
Background. Data on long-term central nervous system (CNS) toxicity associated with efavirenz therapy are scarce, and risk factors remain largely unknown. We aimed to determine whether monitoring the plasma concentration of efavirenz could predict neuropsychiatric adverse events associated with long-term therapy with efavirenz.
Methods. We performed a longitudinal study involving 17 consecutive human immunodeficiency virus (HIV)infected subjects with virological suppression after at least 6 months of antiretroviral therapy with an efavirenz-containing regimen. Efavirenz plasma concentrations were measured at study entry and at different time points through an 18-month study period.
Results.
Median duration of efavirenz therapy before study entry was 18 months (range, 627 months).
Ten (58.8%) of the patients experienced CNS-related adverse effects, ranging from insomnia and abnormal dreams to depression with suicidal ideation.
In 4 (23.5%) of the cases, CNS toxicity led to efavirenz discontinuation.
Mean (± standard deviation) plasma levels were higher for patients experiencing neuropsychiatric symptoms (5.10 ± 2.15 ug/mL vs. 2.79 ± 1.31 ug/mL; P = .024).
A plasma level of 2.74 ug/mL had a sensitivity of 90.9% and specificity of 72% to predict CNS toxicity (area under the curve, 0.839; 95% confidence interval, 0.730.95; P < .0001).
Patients having efavirenz plasma concentrations >2.74 ug/mL at any time point of the study were 5.68 times more likely to experiencing CNS toxicity than were other patients (95% confidence interval, 1.9716.37).
Conclusions.
In patients with HIV infection receiving long-term therapy with efavirenz-containing antiretroviral regimens, CNS toxicity is related to efavirenz plasma levels. Patients achieving higher plasma levels are at increased risk of experiencing neuropsychiatric adverse events.
RESULTS
Subjects. Baseline characteristics of the 17 patients enrolled in the study are shown in table 2. They were receiving antiretroviral therapy with efavirenz in combination with zidovudine and lamivudine (68.7% of patients), stavudine and lamivudine (11.8%), didanosine and lamivudine (11.8%), or nonlamivudine regimens (11.8%). Median time receiving efavirenz therapy before study entry was 18 months (range, 627 months).
Thirteen (76.5%) of the 17 patients completed the 18-month study period with no changes in antiretroviral regimen being made. In 4 (23.5%) of the cases, efavirenz therapy was discontinued during the observation period because of adverse effects. Adherence to antiretroviral therapy was >90% for 16 of the 17 patients. The following concomitant medications were used during the study: omeprazole (3 patients), lorazepam (3), ibuprofen (2), trimethoprim-sulfamethoxazole (2), fenofibrate (1), ciprofloxacin (1), levofloxacin (1), amoxicillin (1), pyrimethamine (1), valacyclovir (1), and cetirizine (1). All patients maintained HIV suppression while receiving efavirenz and the mean (±SD) CD4+ T lymphocyte count increased from 416.9 ± 205.41 cells/mm3 to 526.6 ± 292.55 cells/mm3 (P = .007).
CNS adverse effects.
Overall, 10 patients (58.8%) reported experiencing neuropsychiatric symptoms during the observation period, mostly sleep disturbances. The following CNS adverse effects were recorded: dizziness (6 cases [35.3%]), insomnia or abnormal dreaming (5 [29.4%]), impaired concentration and attention span (2 [11.8%]), depression (2 [11.8%]), obsessive disorder (1 [5.9%]), drowsiness (1 [5.9%]), irritability (1 [5.9%]), and light headedness (1 [5.9%]).
In 6 of 10 patients, neuropsychiatric symptoms were mild (grade 1 on the World Health Organization scale). In 4 (23.5%) of the cases, CNS toxicity was moderate or severe (grade 2 or more on the World Health Organization scale), leading to efavirenz discontinuation at months 6, 8, 11, and 13, respectively, after enrolling in the study. The neuropsychiatric events leading to efavirenz discontinuation were as follows: depression, 2 cases (accompanied by suicidal ideation in 1 case); mood changes with impaired concentration and attention, 1 case; and an obsessive disorder, 1 case. In 2 of the 4 cases, mild neuropsychiatric symptoms (insomnia and light headedness) were present before they developed the event that required discontinuation of therapy. Total duration of efavirenz therapy for patients who discontinued treatment with the drug ranged from 16 months to 34 months.
No statistically significant differences were found in the incidence of neuropsychiatric symptoms according to sex (8 cases among the 14 male patients [57.1%] vs. 2 cases among the 3 female patients [66.7%]; P = .76) or hepatitis C virus (HCV) coinfection (5 cases among the 8 HCV-coinfected patients [62.5%] vs. 5 cases among the 9 nonHCV-coinfected patients [55.5%]; P = .77). Body weight was not significantly different in patients with or without CNS-related adverse effects (median weight [interquartile range], 62.5 [52.975.2] kg vs. 71.5 [55.074.5] kg; P = .64).
CNS-related adverse effects relative to efavirenz plasma levels:
During the study period, a total of 58 efavirenz plasma concentration measurements were performed, with a median of 3 determinations per patient (range, 35 determinations). Blood sampling took place at a median of 12 h after drug intake (range, 913 h). Efavirenz concentrations ranged from 0.62 ug/mL to 12.59 ug/mL, with a mean value (±SD) of 4.12 ± 2.51 g/mL (figure 1). The repeated determinations revealed moderate intrapatient variability (coefficient of variation, 28.29%; range, 5.79%74.30%) over 6 month intervals, and large interpatient variability (coefficient of variation, 60.96%).
Although no significant correlation was found between efavirenz plasma concentrations and body weight, subjects weighing <60 kg tended to have higher efavirenz levels than those weighing >60 kg (mean efavirenz plasma concentration [±SD], 5.01 ± 3.45 g/mL vs. 3.52 ± 1.60 g/mL; P = .07]. No significant differences in efavirenz plasma concentrations were observed between male and female patients (mean efavirenz plasma concentration [± SD], 4.31 ± 2.55 g/mL vs. 3.30 ± 2.26 g/mL; P = .23) nor between HCV-coinfected and non-HCV-coinfected patients (mean efavirenz plasma concentration [±SD], 3.57 ± 1.28 g/mL vs. 4.47 ± 3.00 g/mL; P = .12).
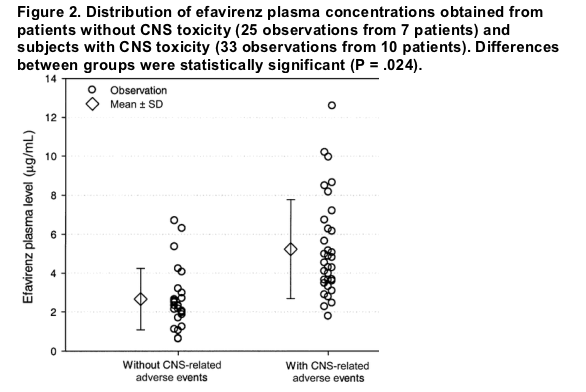
The distribution of efavirenz plasma concentrations according to CNS toxicity is shown in figure 2. Mean (±SD) plasma levels were higher for patients experiencing neuropsychiatric symptoms during the observation period (5.10 ± 2.15 g/mL vs. 2.79 ± 1.31 ug/mL; P = .024). Area under the receiver operating characteristic curve was assessed for different efavirenz plasma levels (figure 3). A level of 2.74 ug/mL had a sensitivity of 90.9% and a specificity of 72% to predict CNS toxicity. The area under the curve was 0.839 (95% CI, 0.730.95; P < .0001). Patients having efavirenz plasma concentrations >2.74 ug/mL at any time point of the study were 5.68 times more likely to present with CNS toxicity (95% CI, 1.9716.37; P = .000001). Ten (83.3%) of the 12 efavirenz plasma levels obtained from the 4 patients requiring drug discontinuation were >2.74 ug/mL. In the multivariate analysis, including efavirenz plasma concentration >2.74 g/mL, body weight, and coinfection with HCV, only efavirenz concentration was significantly associated with CNS toxicity (P < .0001).
DISCUSSION
In this study, we included patients with long-lasting viral suppression who were receiving a typical antiretroviral regimen combining efavirenz with 2 NRTIs (usually zidovudine and lamivudine), a scenario that is often seen in current clinical HIV practice. Unexpectedly, more than one-half of the patients reported neuropsychiatric symptoms during long-term efavirenz therapy (mostly mild sleep disturbances and morning dizziness), and one-quarter of the patients developed delayed CNS toxicity that led to discontinuation of therapy with efavirenz. Of note, all of the discontinuations involved patients who had been treated with efavirenz for >1 year, and 1 of the patients had received the drug for almost 3 years, raising concerns about the delayed neuropsychiatric toxicity of efavirenz.
Most previous studies assessing efavirenz-related CNS toxicity involved patients who had received the drug for shorter periods of time [7, 13]. In a recent cross-sectional study involving 60 patients who received efavirenz for a median of 22 months, a wide variety of neuropsychiatric symptoms were observed in up to 54% of the patients (mostly dizziness, sadness, mood changes, and irritability) [11]. Therefore, the prevalence of persistent neuropsychiatric symptoms in patients receiving long-term therapy with efavirenz may be high.
Plasma concentrations of efavirenz in patients taking part in our study ranged from 0.62 ug/mL to 12.59 ug/mL. Given the long half-life of efavirenz and its bedtime dosage, we used mid-interval sampling, a feasible and convenient way of sampling that has been previously validated [7, 13]. The concentrations obtained were similar to those found in previous studies [7, 13, 14]. In contrast to other investigations, which have reported higher plasma concentrations of NNRTIs in HCV-coinfected patients than in non-HCV-coinfected patients [15, 16], we did not find higher efavirenz levels in the 8 subjects with HCV coinfection who were included in the study.
Patients experiencing neuropsychiatric symptoms during efavirenz therapy usually had drug plasma concentrations >2.74 g/mL. This cutoff point was found to have the best discriminatory power to evaluate the risk of CNS toxicity in our study. Indeed, patients who had efavirenz plasma concentrations above this value at any time point during the study were much more likely than others to report neuropsychiatric symptoms. Conversely, patients with efavirenz concentrations below this value seem to have a low risk for developing CNS-related adverse events during long-term efavirenz therapy.
Few previous studies have addressed the relationship between drug concentrations of efavirenz and CNS toxicity [7, 13, 14]. In the study conducted by Marzolini et al. [7], CNS toxicity was about 3 times more frequent among patients with high efavirenz levels (>4 ug/mL), compared with patients with 1-4 ug/mL [7]. Gallego et al. [13] also found a relationship between efavirenz plasma concentrations and efavirenz-related insomnia and sleep efficiency. Fumaz et al. [11] failed to demonstrate an association between CNS toxicity and efavirenz plasma concentrations in patients receiving long-term efavirenz therapy. As Fumaz et al. [11] acknowledge in the report, a possible explanation for the discordance is the cross-sectional nature of their study, which would have missed those patients with the highest plasma levels who might have previously discontinued efavirenz therapy because of adverse events. In fact, plasma levels found by Fumaz et al. [11] were lower than those found in previous studies, and a small proportion of their patients had efavirenz levels >4 ug/mL. Because plasma drug levels were measured only once, intrapatient variability over time may have been another potential caveat of the study.
Our study was specifically designed to address the hypothesis that patients who experience long-term CNS toxicity during treatment with efavirenz might be those with higher plasma exposure to efavirenz. To have a more reliable measure of overall drug exposure, efavirenz plasma concentrations were measured several times throughout the study period. The study results confirm that CNS toxicity associated with long-term therapy with efavirenz is related to efavirenz plasma levels, and they suggest that patients who achieve higher plasma levels may be at increased risk of developing long-term delayed neuropsychiatric adverse events. Interindividual differences in metabolism may, in part, explain susceptibility to CNS adverse effects associated with efavirenz therapy. Recent data from Adult AIDS Clinical Trials Group study A5097s suggest differences in efavirenz exposure between different racial and ethnic populations [10]. In this study, a cytochrome P450-2B6 allelic variant that was more common in black persons than in white persons was associated with significantly greater efavirenz exposure and acute CNS symptoms during HIV therapy [10]. The lack of representation of nonwhite populations in our study did not allow us to compare efavirenz levels among different racial or ethnic groups.
The relationship between CNS-related adverse effects and efavirenz plasma levels opens a door to investigate dosage individualization based on plasma concentration monitoring as a way to optimize management of efavirenz therapy. Therapeutic drug monitoring might be particularly useful for patients with limited therapeutic options and for those experiencing chronic "minor" CNS disturbances (e.g., sleep abnormalities) during efavirenz therapy. An improvement of the symptoms after a dosage reduction is theoretically possible, and there is anecdotal experience suggesting that this improvement may indeed occur [17].
A major concern when adjusting drug dosages of antiretroviral drugs to avoid toxicity may be compromising the virologic efficacy of the drug. Efavirenz plasma concentrations have been shown to influence the virological response to NNRTI-based regimens [7], but limited data exist on the desirable plasma level for achieving virological success. According to previous data, the efavirenz plasma level should be >1 g/mL to provide HIV-1 suppression [7,14]. In our study, most patients achieved concentrations well above that level, and indeed, all of them maintained complete virological suppression while receiving efavirenz therapy. Therefore, if 1 g/mL is considered to be an appropriate target concentration, there may be room for dose reduction to avoid toxicity in selected cases, especially in NNRTI-naive patients with long-lasting viral suppression. However, the situation may be different in pretreated patients, for whom high concentrations of efavirenz may be needed to reach viral suppression. This may be the case for patients experiencing treatment failure with nevirapine, for whom higher efavirenz plasma levels (>3 g/mL) may be associated with higher chances of virological success [18].
Our study has limitations. The main limitation relates to the small sample size, which limits the identification of factors other than efavirenz drug levels (e.g., age, sex, duration of antiretroviral therapy, concurrent use of certain antiretroviral agents, psychological factors, and HCV coinfection) [19] that might also increase the risk of CNS-related neuropsychiatric symptoms. We studied only patients who were highly adherent to their treatment regimens with long-lasting viral suppression while receiving efavirenz plus 2 NRTIs, and therefore our data may not be applicable to all patients with HIV infection who receive efavirenz. Neuropsychiatric adverse events might be different in other patient populations. Despite these limitations, the association we found between CNS toxicity and efavirenz plasma concentrations is robust and consistent with previous data.
In summary, our study provides indirect evidence for a higher risk of CNS-related adverse events among patients with higher efavirenz plasma concentrations who receive long-term antiretroviral therapy with efavirenz-containing regimens. Monitoring of plasma levels should allow patients with higher efavirenz plasma levels who are, consequently, at increased risk for neuropsychiatric adverse events to be detected. Future studies are needed to assess whether therapeutic drug monitoring will result in a decrease in the percentage of patients developing neuropsychiatric abnormalities while receiving efavirenz-containing regimens with no loss of efficacy.
METHODS
Study population.
Patients were recruited into the study at the outpatient HIV clinic of a university hospital (Hospital General Universitario de Elche, Alicante, Spain). Eligible patients were all HIV-infected adults (age, 18 years) treated in the clinic during a 3-month period who were clinically stable and had virological suppression (HIV RNA level, <50 copies/mL) while receiving combination antiretroviral therapy with efavirenz at the standard dose (600 mg once per day at bedtime) and 2 nucleoside reverse-transcriptase inhibitors (NRTIs) for at least 6 months. Other inclusion criteria were a good adherence to antiretroviral drugs (95%) and the absence of active opportunistic infection or acute illnesses. Patients were not to have had a previous history of depression or other mental disorders and were not to be receiving psychiatric medication or methadone at the time of recruitment. The local ethics committee approved the study. All patients meeting the inclusion criteria were asked to participate in the study. All subjects agreed to participate and gave their informed consent before enrollment. Decisions on medical management during the study were made by attending clinicians in charge of the patients according to standard-of-care practice, without knowing the pharmacokinetic results.
Clinical and laboratory evaluations.
Each patient was evaluated at study entry and at 3-month intervals during an 18-month study period. Demographic and clinical data relating to HIV infection, antiretroviral treatment received previously or during the study, and concomitant medications were recorded at baseline and at each visit. Drug-related adverse effects and adherence to therapy were recorded at each visit. CNS-related adverse effects were assessed using a semistructured interview that included questions exploring common presumptive efavirenz-related adverse effects (table 1). Toxicity was graded according to the World Health Organization toxicity scale. Treatment adherence was evaluated with the validated Simplified Medication Adherence Questionnaire [12]. Safety blood tests were performed at each visit, and CD4+ T lymphocyte count (measured by flow cytometry) and plasma HIV RNA level were determined (measured with Roche Amplicor software, version 1.5 [Roche Diagnostics], with a lower limit of detection of 50 copies/mL of plasma).
Pharmacokinetic evaluations.
Efavirenz plasma concentrations were measured at study entry and at different time points during the 18-month study period (months 0, 3, 6, 12, and 18). Blood samples were collected in heparinized tubes at 9 A.M., after an overnight fast. Plasma was isolated by centrifugation on the same day and was stored immediately at -70C until analyzed. Efavirenz concentrations in plasma were measured using a validated high-performance liquid chromatography assay. In brief, a 0.5-mL plasma aliquot was combined with a 20-uL volume of working internal standard solution at a concentration of 500 ug/mL, 1.0 mL of 0.5-M Na2 CO3, and 6 mL of ethyl acetate/hexane (1 : 1 vol/vol) in a 15-mL glass culture tube. The organic layer was transferred to a conical glass centrifuge tube and evaporated to dryness. The samples were sequentially reconstituted with aliquots of acetonitrile and water (1:1 vol/vol) and transferred to autosampler vials for high-performance liquid chromatography UV analysis. A 20-uL injection volume was used throughout the analytic validation and batch analysis. The unknown concentrations were computed from the unweighted linear least-squares regression of the peak area ratio against the concentration for the calibration curve. The high-performance liquid chromatography UV system consisted of a Gold Beckman chromatograph and a 20-uL Rheodine injector. The detector output UV (Gold Beckman, model 166) was operated at a wavelength of 205 nm. Chromatographic separation was accomplished on a Kromasil 100 C18 5-mc 25-mm X 0.46-mm column. The mobile phase contained a mixture of acetonitrile, methanol, and 0.01-M tetramethylammonium perchlorate in 0.1% aqueous trifluoroacetic acid (40 : 5 : 55 ratio) at a constant flow rate of 1.0 mL/min at 36C. The concentration range curve was prepared at 0.115 mcg/mL. The lower limit of quantification of the assay was 0.1 mcg/mL. The intra- and interassay variabilities were <5%.
Statistical analysis. SPSS for Windows, version 11 (SPSS), was used for data management and statistical analysis. Descriptive statistics were computed by standard methods. Student's t test was used to compare efavirenz plasma levels obtained from patients who experienced neuropsychiatric symptoms with those obtained from patients who did not. Receiver operating characteristic curves were used to select the cutoff point of efavirenz plasma level with the best discriminatory power between patients with CNS toxicity and those without CNS toxicity. To calculate the relative risk, we constructed 2 X 2 contingency tables and determined the 95% CI. The multivariate analysis of variables potentially associated with CNS toxicity was performed by stepwise logistic regression. A 2-tailed P value of .05 was considered to be significant.
|
|
| |
| |
|
|
|