| |
Reyataz- body changes, metabolics -48 weeks
|
| |
| |
Body Fat and Other Metabolic Effects of Atazanavir and Efavirenz, Each Administered in Combination with Zidovudine plus Lamivudine, in Antiretroviral-Naive HIV-Infected Patients
Clinical Infectious Diseases Jan 15 2006;42:000
Joseph G. Jemsek,1 Eduardo Arathoon,4 Massimo Arlotti,7 Carlos Perez,6 Nestor Sosa,5 Vadim Pokrovskiy,8 Alexandra Thiry,2 Michael Soccodato,2 Mustafa A. Noor,3 and Michael Giordano2
1Jemsek Clinic, Huntersville, North Carolina; 2Bristol-Myers Squibb, Wallingford, Connecticut; 3Bristol-Myers Squibb, Princeton, New Jersey; 4Hospital General San Juan de Dios, Guatemala, Guatemala City; 5Consultorio Royal Center, Panama City, Panama; 6Hospital Clinico de La Pontificia Universidad Catolica, Santiago, Chile; 7Ospedale degli Infermi, Rimini, Italy; and 8Federal AIDS Center, Moscow, Russia
(See the editorial commentary by Dube following the study report below)
ABSTRACT
Background. Protease inhibitor treatment of human immunodeficiency virus (HIV)infected individuals has been linked to the development of lipodystrophy. The effects of atazanavir on body fat distribution and related metabolic parameters were examined in antiretroviral-naive patients.
Methods. HIV-positive patients with CD4 cell counts >100 cells/mm3 were randomized to 1 of 2 treatment arms: (1) atazanavir, 400 mg given once daily, plus efavirenz placebo; or (2) efavirenz, 600 mg given once daily, plus atazanavir placebo; each drug was administered with fixed-dose zidovudine (300 mg) and lamivudine (150 mg) given twice daily, and patients were treated for at least 48 weeks. Fat distribution measurements (visceral adipose tissue [VAT], subcutaneous adipose tissue [SAT], and total adipose tissue [TAT], as measured by computed tomography; and appendicular fat, truncal fat, and total fat levels, as measured by dual-energy X-ray absorptiometry), metabolic measurements (cholesterol and fasting triglyceride levels), and measurements of insulin resistance (fasting glucose and fasting insulin levels) were made at baseline and at week 48 of treatment for a subgroup of 111 atazanavir recipients and 100 efavirenz recipients.
Results. Atazanavir and efavirenz treatments resulted in minimal to modest increases in fat accumulation, as measured by VAT, SAT, TAT, appendicular fat, truncal fat, and total fat levels; results were comparable in both arms. In addition, atazanavir was associated with none of the metabolic abnormalities seen with many other protease inhibitors. See tables of results below.
Conclusions. Use of atazanavir for 48 weeks neither resulted in abnormal fat redistribution in antiretroviral-naive patients nor induced other metabolic disturbances commonly associated with HIV-related lipodystrophy. Longer-term assessments (e.g., at 96 weeks) will be important to confirm these findings.
METHODS
Study
The main phase III study was a multinational, randomized, double-blind comparative trial in 810 patients at 91 study centers in Europe, Asia, Africa, and North, Central, and South America. The trial has been described elsewhere [23]. In brief, for inclusion in the study, patients had to be aged 16 years and have a baseline plasma HIV RNA level of 2000 copies/mL and a baseline CD4 cell count of 100 cells/mm3 (or 75 cells/mm3 with no prior history of any AIDS-defining diagnosis). Patients were excluded from participation if they had received prior antiretroviral therapy (defined as treatment for >30 days with nucleoside reverse-transcriptase inhibitors and/or >7 days with nonnucleoside reverse-transcriptase inhibitors or protease inhibitors) or any antiretroviral therapy within 30 days prior to screening. Randomization was performed to 1 of 2 treatment arms on a 1 : 1 basis: (1) atazanavir, 400 mg given once daily, plus efavirenz placebo; or (2) efavirenz, 600 mg given once daily, plus atazanavir placebo; each was given in combination with fixed-dose zidovudine and lamivudine (300 mg and 150 mg, respectively, twice daily).
Metabolic Substudy
Objectives.
The primary objective of the metabolic substudy was to assess the effect of atazanavir on abdominal fat distribution, as measured by change in visceral adipose tissue (VAT) levels from baseline to week 48. Secondary objectives of the study included assessment of the effect of atazanavir on (1) abdominal fat distribution, as measured by changes from baseline in subcutaneous adipose tissue (SAT) level, total adipose tissue (TAT) level, and ratios of SAT level to VAT level and of VAT level to TAT level at week 48; (2) appendicular fat level, as measured by changes from baseline in total fat of the appendages and the percentage of appendicular fat relative to total body fat at week 48; and (3) truncal fat level, as measured by changes from baseline in total truncal fat level and the percentage of truncal fat relative to total body fat at week 48. Relative differences between atazanavir- and efavirenz-containing regimens were to be assessed. In addition, also described were the effects of the 2 treatments on metabolic parameters, including total cholesterol, fasting low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, fasting triglyceride, and non-HDL cholesterol level and insulin resistance, as measured by changes in fasting glucose and fasting insulin levels from baseline through week 48.
Patient selection.
Sites were selected to participate in the substudy on the basis of the availability of DEXA and CT scan equipment and investigator interest and ability. Patient participation was voluntary and was offered to all those in the main phase III study who were enrolled at participating sites, with the exception of those who had uncontrolled hypercholesterolemia, a history of endocrine diseases, triglyceride levels of >750 mg/dL, or uncontrolled hypogonadism or who were receiving agents associated with metabolic changes or fat distribution. Patients who were receiving lipid-lowering agents, growth hormone, megestrol acetate, anabolic agents, anticytokine agents, ketoconazole, or systemic glucocorticosteroids within 6 months of study entry were also excluded.
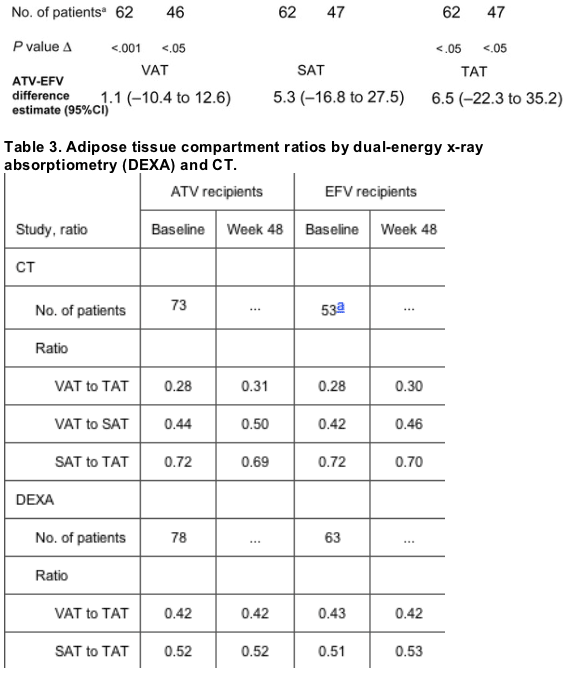
A total of 211 patients from study centers in 7 countries (2 in Europe, 1 in North America, 3 in Central America, and 1 in South America) participated in the substudy. A total of 111 patients received atazanavir, and 100 received efavirenz.
Fat distribution measurements.
All DEXA and CT measurements were obtained prior to any medical intervention for increased cholesterol or triglyceride levels. SAT, VAT, and TAT levels were measured by CT scan in square centimeters with a single slice at L4L5; total body fat, total appendicular fat, and total truncal fat levels were measured by DEXA scan in kilograms. DEXA and/or cross-section CT scans were performed at baseline and at week 48. Patients who discontinued the study before week 48 underwent DEXA and CT at their final visit, provided that visit was at or after week 24, and these data served as their week 48 measurements. All DEXA and CT scan results were submitted to a central reader for interpretation.
Analyses relating to fat distribution included patients treated with atazanavir or efavirenz who had DEXA and CT assessments at baseline and after week 24 obtained before initiation of lipid-lowering therapy. Primary analyses included only patients treated with atazanavir. However, for reference, descriptive analyses of changes in fat concentrations measured with DEXA or CT methodology were also conducted for patients treated with efavirenz. In the primary analysis, VAT summary statistics were computed for baseline, week 48, and change from baseline. The difference in the mean change from baseline in VAT level at week 48 was computed along with a 2-sided 95% CI based on a t test. Summary statistics were also computed for baseline, week 48, and change from baseline for the secondary outcome measures of SAT level, TAT level, appendicular fat level, percentage of appendicular fat relative to total body fat, total truncal fat level, percentage of truncal fat relative to total body fat, ratio of SAT level to VAT level, and ratio of VAT level to TAT level. Differences between treatment regimens (atazanavir and efavirenz) were computed with 95% CIs for CT measurements (i.e., VAT, SAT, and TAT levels) and DEXA measurements (i.e., appendicular, truncal, and total body fat levels).
In addition, mean levels at baseline and week 48 were assessed for total cholesterol, fasting LDL cholesterol, HDL cholesterol, non-HDL cholesterol, fasting triglycerides, fasting glucose, fasting insulin, and fasting Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). Mean changes from baseline at week 48 for these parameters were also assessed. The mean ratio of total cholesterol level to HDL cholesterol level was assessed at baseline and week 48.
INTRODUCTION
Since the late 1990s, increasing numbers of HIV-infected patients have been found to have lipodystrophy. HIV-related lipodystrophy is characterized by disturbances in the normal distribution of body fat, including peripheral lipoatrophy of the face, limbs, and buttocks and/or fat accumulation in the abdomen and breasts and at the base of the neck [1, 2]. Clinical estimates place the incidence of HIV-related lipodystrophy in the range of 13%-83%, depending on the patient population [1, 37], and patients can demonstrate some or all of the features of the disorder. In one study [5], 49% of patients had >1 sign of fat maldistribution; central adiposity was the most common feature noted, followed by thinning of the extremities and buttocks, sunken cheeks and other facial changes, and enlargement of the dorsocervical fat pad. Alterations in fat distribution can occur in conjunction with a variety of metabolic disorders, including dyslipidemia [1, 3, 4, 7], hyperglycemia [3, 8], insulin resistance [9-11], and diabetes [9]. Collectively, peripheral lipoatrophy, central adiposity, dyslipidemia, and insulin resistance are known as "lipodystrophy syndrome" [7].
HIV-related fat accumulation is most frequently associated with protease inhibitor therapy [24, 7, 12, 13], although it is also seen, to a lesser extent, with the use of nucleoside reverse-transcriptase inhibitors [5, 6, 14, 15]. Attempts to precisely quantify the effects of antiretroviral therapy on fat distribution have been limited by the lack of criteria for diagnosing and rating the severity of lipodystrophy [7], and most investigations have been based on patient self-reports and/or clinical observation. However, studies using objective measures, such as dual-energy X-ray absorptiometry (DEXA) and CT, have confirmed clinical observations demonstrating the involvement of protease inhibitors in the development of lipodystrophy. As diagnostic tools, DEXA scans are suitable for estimating overall amounts of appendicular and truncal fat, whereas CT scans provide accurate estimations of subcompartment distribution of subcutaneous and visceral fat [16]. As such, DEXA scans have documented a greater severity of peripheral lipoatrophy associated with nelfinavir therapy than with efavirenz therapy [15], and abdominal CT scans have shown that indinavir is associated with the accumulation of intraabdominal (visceral) fat [2].
To varying degrees, the majority of protease inhibitors have been associated with the metabolic disturbances and/or morphological alterations that constitute lipodystrophy syndrome [9, 14]. Recently, however, a once-daily azapeptide protease inhibitor, atazanavir, has become available that does not result in the lipid level elevations characteristic of other protease inhibitors [17, 18]. In addition to clinical data demonstrating a favorable safety profile in terms of lipid effects, in vitro studies have shown that atazanavir does not increase lipid synthesis in hepatocytes or suppress lipid synthesis in adipocytes [19]. Moreover, unlike other protease inhibitors tested, atazanavir does not inhibit insulin-stimulated glucose transport via glucose transporter (GLUT)-4 in vitro, a measurement of insulin resistance [20]. A placebo-controlled clinical study that compared the effects of atazanavir with those of lopinavir/ritonavir on insulin-stimulated glucose disposal and glycogen storage rates showed that atazanavir had no effect on either of these parameters, nor did it induce insulin resistance. In contrast, lopinavir/ritonavir reduced insulin-stimulated glucose disposal and the rate of glycogen storage and induced insulin resistance [21]. In a smaller, uncontrolled open-label study by Lee and colleagues [22], statistically and clinically significant induction of insulin resistance was not detected by euglycemic hyperinsulinemic clamp, but it was detected by oral glucose tolerance testing.
The benign effects of atazanavir on metabolic parameters suggest that the drug may also have a reduced ability to induce lipodystrophy. We report the effects of atazanavir on body fat distribution and other metabolic parameters in antiretroviral-naive HIV-infected patients. Patients were recruited from a larger phase III clinical trial (BMS AI424-034) for the reported metabolic study that compared the efficacy and safety of atazanavir and efavirenz, each administered in combination with fixed-dose zidovudine and lamivudine.
RESULTS
Patient demographic characteristics and baseline data.
Patients in the 2 treatment groups had comparable baseline characteristics (table 1). As a whole, the substudy population had a median age of 30 years and was predominantly male (73%). Most of the patients were Hispanic or white (48% and 45%, respectively) and from South America or Europe (49% and 42%, respectively). Prior injection drug use was reported for 11% of the participants. Three percent of patients had a prior Centers for Disease Control and Prevention Class C AIDS-defining illness. The median plasma HIV RNA level and CD4 cell count were 4.75 log10 copies/mL and 328 cells/mm3, respectively. The median body mass index (calculated as weight in kilograms divided by the square of height in meters) was 23.2, indicating a generally lean population.
The substudy population differed racially and geographically from the population of the main study, reflecting the locations of the substudy sites. In the main study, fewer patients were Hispanic and white (37% and 33%, respectively), and fewer patients were from South America and Europe (34% and 28%, respectively). The substudy also differed from the main study in that 27% of the participants were female, compared to 35% in the main study.
Patients who had paired CT and DEXA scan results (at baseline and 48 weeks) were included in the analyses of changes from baseline to week 48; a total of 62 of 111 patients in the atazanavir arm and 47 of 100 patients in the efavirenz arm had paired CT scan results, and 77 of 111 patients in the atazanavir arm and 63 of 100 patients in the efavirenz arm had paired DEXA scan results.
CT scan results.
Although baseline SAT and TAT scores were slightly higher in the atazanavir group than in the efavirenz group, the 2 treatment arms were generally comparable (table 2). At week 48, modest but statistically significant increases of 15.3 cm2 (increase of 33%) and 14.1 cm2 (increase of 23%) were noted in VAT level and 28.0 cm2 (increase of 21%) and 21.6 cm2 (increase of 10%) were noted in TAT level for the atazanavir and efavirenz arms, respectively (figure 1). Smaller increases that were not statistically significant were observed in SAT level for both atazanavir- and efavirenz-treated patients. Mean percentage increases from baseline in VAT, SAT, and TAT were higher in the atazanavir group than in the efavirenz group. Changes from baseline at week 48 in mean ratios of VAT level to TAT level, VAT level to SAT level, and SAT level to TAT level, however, were minimal, yet they were comparable between treatment regimens (table 3).
Table 2. Adipose tissue compartments by dual-energy x-ray absorptiometry (DEXA) and CT at baseline and 48 weeks between groups.
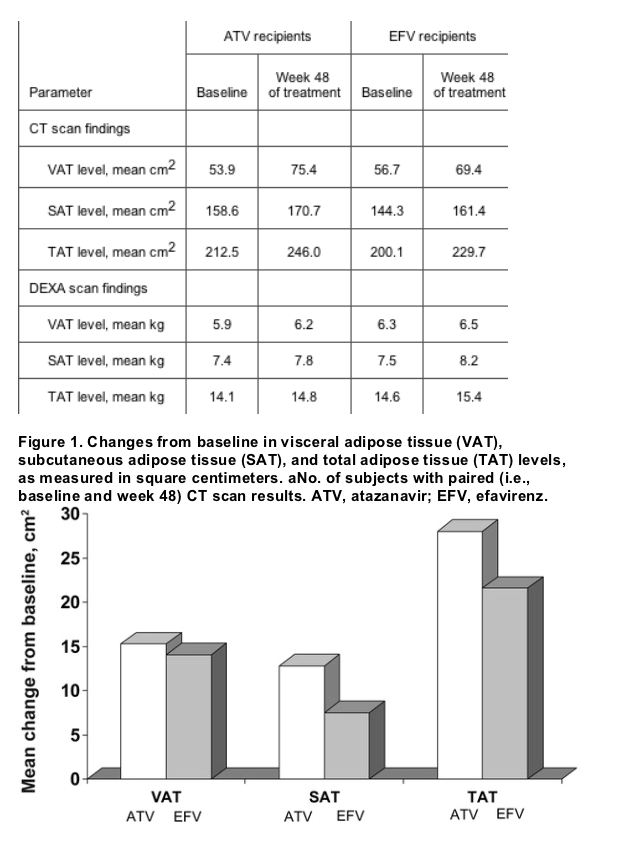
DEXA scan results.
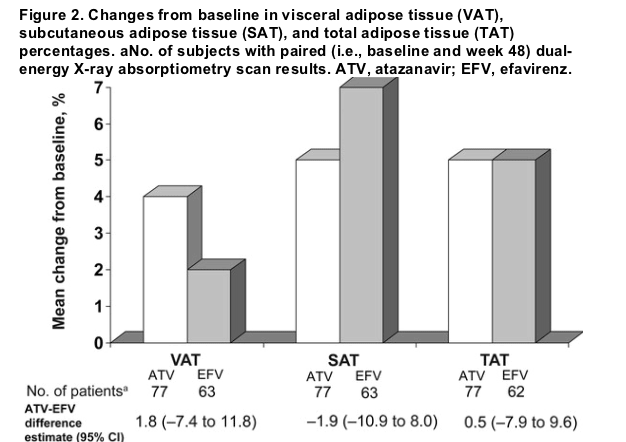
The baseline appendicular, truncal, and total body fat measurements were comparable between treatment groups (table 2). Mean percentage changes from baseline at week 48 in these parameters were not significant, yet they were comparable in the atazanavir and efavirenz arms (figure 2). No significant changes from baseline were observed in the ratios of appendicular fat to total fat or of truncal fat to total fat in either treatment group (table 3).
Weight. Baseline measurements in the 2 groups were similar (mean weight, 67 kg for the atazanavir group and 68 kg for the efavirenz group). At week 48, mean increases in body weight were 2 kg for the atazanavir group and 0 kg for the efavirenz group.
Metabolic measurements.
Atazanavir was not associated with any statistically significant increase in LDL cholesterol level or total cholesterol level (table 4). At week 48, patients receiving atazanavir had a 1% increase in total cholesterol level and no increase in fasting LDL cholesterol level, compared with a 20% increase in total cholesterol level and a 17% increase in fasting LDL cholesterol level observed in those treated with efavirenz. Fasting triglyceride and non-HDL cholesterol levels were reduced by 6% and 1%, respectively, in the atazanavir group, compared with respective increases of 16% and 18% in patients receiving efavirenz. The HDL cholesterol level increased by 11% in the atazanavir group and 21% in the efavirenz group.
Table 4. Metabolic laboratory assessments among atazanavir (ATV) and efavirenz (EFV) recipients.
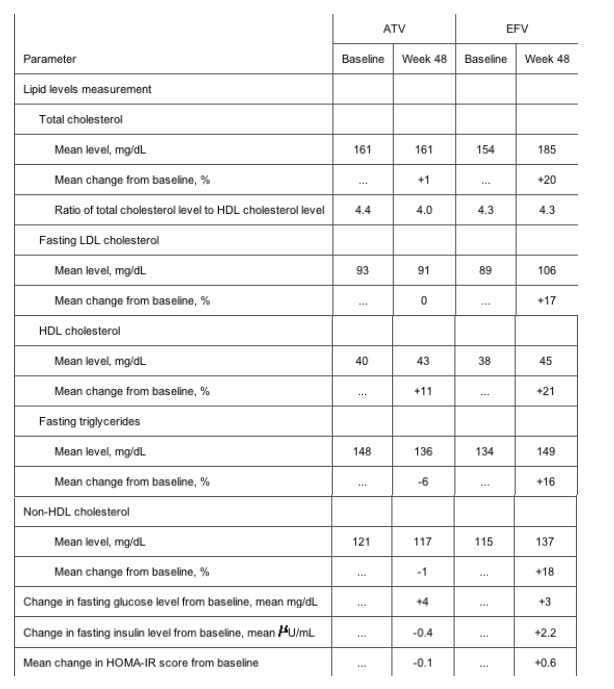
The changes in fasting glucose levels at week 48 were comparable between the 2 treatment groups (table 4). There was a slight decrease in the fasting insulin level in the atazanavir arm, compared with a slight increase in the efavirenz arm. Homeostasis model assessment of insulin resistance [24] revealed a small decrease for the index in atazanavir-treated patients from baseline at week 48; the minimal increase observed for efavirenz-treated patients was not statistically significant.
DISCUSSION
In this study, 48 weeks of therapy with atazanavir plus fixed-dose zidovudine and lamivudine resulted in changes in body fat that were consistent with immune reconstitution and weight gain caused by improved health status. Mean absolute and percentage changes from baseline in appendicular, truncal, and total fat levels in atazanavir-treated patients were comparable to those seen in efavirenztreated patients. Increases observed in VAT, SAT, and TAT levels from baseline to week 48 were modest in both treatment groups. No meaningful changes from baseline were observed on either regimen in the ratios of appendicular fat to total fat or of truncal fat to total fat. Adipose tissue ratios also remained relatively constant, consistent with DEXA scan results. Particularly important, the ratio of VAT level to TAT levela recognized marker of central adiposityremained stable, at 0.3 in both treatment groups.
Few data exist with respect to DEXA and CT measurements in control subjects that would allow individual patients to be classified as having normal or abnormal fat distribution, and there is a large amount of variation in body fat mass and abdominal girth in the general population [1, 16]. However, in a study of indinavir-treated patients, those complaining of increased abdominal girth had a mean ratio of VAT level to TAT level of 0.70, whereas those who had been treated with indinavir for a mean of 9.7 months but were asymptomatic had a mean ratio of 0.59. HIV-infected patients who had not been treated with indinavir had a mean ratio of VAT level to TAT level of 0.40 [2]. The differences in this ratio between each of the indinavir-treated groups and the control group were statistically significant; however, no significant difference was observed between the indinavir-treated groups. One limitation of this study is that the assessment was only conducted through week 48, which may be too soon to observe manifestations of fat redistribution. Additionally, some cohort studies have suggested the contributions of baseline immunologic status to fat redistribution [5]. This study only assesses patients with a baseline CD4 cell count of 100 cells/mm3 who may be at higher risk for lipoatrophy. This exclusion may limit the ability to observe some manifestations of fat redistribution potentially related to baseline immunologic status.
In line with previous findings and in contrast with results for other protease inhibitors, atazanavir did not result in significant increases in total cholesterol, fasting LDL cholesterol, non-HDL cholesterol, or fasting triglyceride levels. In addition, atazanavir did not result in substantive increases in fasting glucose, fasting insulin, or insulin resistance indices.
Although the etiology of HIV-related lipodystrophy has not been fully elucidated, a variety of mechanisms have been proposed. On the basis of the partial homology between HIV protease and LDL receptorrelated protein, a protein that promotes the storage of triglycerides in adipocytes, it has been suggested that inhibition of LDL receptorrelated protein by protease inhibitors may lead to hypertriglyceridemia, which may in turn contribute to the morphological redistribution of fat characteristic of protease inhibitorinduced lipodystrophy [2527]. HIV protease also shares partial homology with cytoplasmic retinoic acidbinding protein type-1, a protein involved in an early step in the inhibition of adipocyte apoptosis and the up-regulation of adipocyte proliferation and differentiation [26, 27]. Conceivably, by inhibiting the function of cytoplasmic retinoic acidbinding protein type-1, protease inhibitors may cause increased apoptosis and decreased differentiation and proliferation of peripheral adipocytes [25, 27]. Possible consequences of these effects include the inability of fatty tissue to clear circulating triglycerides and the release of lipids into the blood by apoptotic lipocytes [2527].
Recently, a complex model linking lipid, insulin, and fat distribution disturbances has been proposed [28]. In this model, the inhibitory effects of protease inhibitors on both the differentiation of proliferating preadipocytes to mature adipocytes and the proteasomal degradation of sterol regulatory elementbinding protein (SREBP)-1, a key lipogenic transcription factor, result in increased levels of SREBP-1 [1, 26, 29]. This increase in SREBP-1 leads to increased levels of cholesterol and triglycerides, which in turn may increase insulin resistance [1, 26]. Protease-induced inhibition of GLUT-4 may further exacerbate the increase in insulin resistance [1, 20]. Increased levels of SREBP-1 and perhaps the inhibition of GLUT-4 may also contribute to the development of peripheral lipoatrophy, which has the effect of further increasing insulin resistance [1, 30]. In turn, increased insulin resistance may fuel greater peripheral lipoatrophy [20, 31]. In a study comparing the majority of available protease inhibitors, atazanavir demonstrated the least interference with GLUT-4, triglyceride synthesis, and proteasomal activity, providing a possible explanation for the results obtained in the present study [20].
Atazanavir and efavirenz were associated with proportional effects on body fat distribution through 48 weeks of treatment of previously antiretroviral-naive patients. Minimal-to-modest increases in fat level were consistently noted in both groups in all compartments. Fat increases observed were consistent with successful HIV treatment and a return to health. In addition to its lack of effect on fat distribution, atazanavir did not induce other metabolic disturbances commonly associated with HIV lipodystrophy. Additional long-term comparative data are needed for treatment-experienced patients. In addition, longer-term (e.g., 96-week) follow-up of the present treatment-naive cohort is needed to confirm the findings of this study.
Financial support. Bristol-Myers Squibb.
EDITORIAL
HIV-Associated Lipoatrophy: What Are the Kinder, Gentler Agents?
Michael P. Dube
Division of Infectious Diseases, Indiana University, Indianapolis
Lipoatrophy associated with antiretroviral treatment of HIV infection is a common and serious problem that is associated with significant aesthetic and metabolic derangements [1]. Clearly, therapy with thiazolidenediones or switching of therapy to nucleoside reverse-transcriptase inhibitors is suboptimal for addressing severe established lipoatrophy, so when possible, it is preferable to avoid or minimize this complication altogether by choosing the kinder, gentler antiretroviral agents. Because it is not clear whether fat accumulation, such as abdominal obesity, breast enlargement, and buffalo hump, is a complication associated with the use of particular drugs, I will limit my comments to the more definitively antiretroviral treatmentrelated morphologic complication of lipoatrophy.
Multiple lines of evidence from observational and randomized studies implicate nucleoside reverse-transcriptase inhibitors, particularly stavudine, in lipoatrophy. A recent randomized trial that compared stavudine versus tenofovir (either given in combination with lamivudine and efavirenz) reported greater limb fat levels (as determined by dual-energy X-ray absorptiometry [DEXA]) among tenofovir recipients at weeks 96 and 144 [2]. However, this study did not include baseline DEXA findings and thus could not evaluate the longitudinal patterns of fat changes over time or compare results during treatment with pretreatment status. Thus, it was not clear whether the overall pattern was one of gain or loss over time in this study, but the inferiority of stavudine was clear. In a substudy of a prospective, randomized trial that compared stavudine plus didanosine with zidovudine plus lamivudine (both given in combination with nelfinavir and/or efavirenz), subjects assigned to receive zidovudine and lamivudine had an increase in limb fat level from baseline (as determined by DEXA) of 4% at 64 weeks, whereas subjects assigned to receive stavudine and didanosine had a decrease of nearly 17% (P < .001, by between-groups comparison for overall change from baseline) [3]. Although the slight increase in limb fat level from the baseline level with zidovudine and lamivudine assignment at 64 weeks in this study is encouraging, longer-term data are needed to assess longer-term outcomes [4]. Regardless, differences between nucleoside reverse-transcriptase inhibitors are becoming clearer. Studies that compare tenofovir-, zidovudine-, and abacavir-based combination regimens are anxiously awaited. Existing clinical data are inadequate to speculate whether there are differences between those agents with regards to lipoatrophy.
HIV-1 protease inhibitors, when given in combination with nucleoside reverse-transcriptase inhibitors, may also be capable of contributing to lipoatrophy. There are conflicting data from observational studies with regard to the independent contribution of protease inhibitors to the development of lipoatrophy [4, 5]. Substitution of nonnucleoside reverse-transcriptase inhibitors for protease inhibitors has not been effective for treatment of established lipoatrophy. However, one randomized prospective study revealed greater limb fat loss with nelfinavir treatment, compared with efavirenz treatment, over 64 weeks [3]. However, the magnitude of the protease inhibitor effect in this study was smaller than the effect of nucleoside assignment.
In this issue of Clinical Infectious Diseases, in a substudy of a randomized trial of subjects with a CD4 cell count of >100 cells/mm3 whose initial antiretroviral therapy consisted of zidovudine and lamivudine plus either efavirenz or atazanavir [6], Jemsek et al. [7] report no evidence of lipoatrophy at 48 weeks of treatment. With both combinations, there was a small increase from the baseline level in both abdominal subcutaneous and limb fat levels that was not statistically significant, and there was an increase in the visceral fat level noted by CT. Although analysis of between-groups differences was not a primary objective, there appeared to be very similar effects with both efavirenz and atazanavir with regard to total and regional body fat changes. Consistent with the main study's results [6], the new azapeptide protease inhibitor atazanavir had no significant effects on glucose or lipid measurements.
But do these results suggest a differential effect of atazanavir, compared with other protease inhibitors, on body fat changes? The metabolic effects of different protease inhibitors are often divergent; for example, indinavir causes early insulin resistance with relatively few lipid effects, and amprenavir causes significant lipid changes without early effects on insulin resistance [8]. Nelfinavir did not induce early insulin resistance, but it did have significant effects on lipids and limb fat [3]. Thus, a lack of atazanavir-based effects on lipid and glucose metabolism may not necessarily translate into kinder, gentler effects on subcutaneous fat. Unfortunately, a 48-week follow-up period may not be sufficient to detect more-subtle or delayed deleterious effects [4]. The time course of limb fat changes in initially antiretroviral-naive patients involves an early increase during the first 1632 weeks of therapy. This increase occurs regardless of which drugs are used and is followed by variable stability or decrease over time [3, 4], which depends, at least in part, on the drugs being used [3]. This early increase in limb fat level, which may be more pronounced in patients with advanced HIV disease, can be lost as early as 4864 weeks after the initiation of therapy. For example, I was a researcher in a study of regimens that contained didanosine and stavudine; in this study, the limb fat level had rapidly decreased at week 48 from week 16 peak values and was significantly lower than the level associated with zidovudine-lamivudinecontaining regimens, but the level did not become significantly lower than the baseline level until week 64 [3]. Because no DEXA scans were included early in the study (i.e., weeks 1624) by Jemsek et al. [7], it is possible that the limb fat findings reported at week 48 represented values that were significantly decreasing from earlier peak values that were not captured.
Although lower nadir CD4 cell count has not been confirmed as a risk factor for limb fat loss in prospective studies with serial longitudinal assessments, it and a number of other nondrug-related risk factors may also contribute to the risk of lipoatrophy [5]. Jemsek et al. [7] excluded subjects with a CD4 cell count of <100 cells/mm3, thus possibly excluding patients who were at the greatest risk for lipoatrophy. To properly attribute a lack of lipoatrophy to atazanavir use, it will be important to study subjects for a longer period of time and to include data on subjects with lower CD4 cell counts.
The results reported by Jemsek et al. [7] are encouraging, particularly given that the effects on peripheral fat with the newer, glucose/lipid-friendly protease inhibitor atazanavir were comparable to those seen with efavirenz. Longer-term follow-up studies (i.e., those with a duration of >48 weeks) with a full range of entry CD4 cell counts are needed, as are studies of the body fat effects of ritonavir-boosted atazanavir therapy and direct comparisons with other protease inhibitors, before any unique peripheral fat-sparing effects of atazanavir can be confirmed.
|
|
| |
| |
|
|
|