| |
HIV-related Lymphoma Survival Increasing
|
| |
| |
"....The use of HAART has led to a dramatic decline in AIDS-related illnesses and a remarkable improvement in overall survival among patients with AIDS.6 Likewise, there has also been significant improvement in the prognosis of patients with ARL, such that the median overall survival of these patients now approaches that of patients with de novo aggressive non-Hodgkin's lymphoma (NHL)... It is conceivable that better immune reconstitution through the use of HAART has reduced the risk of serious intercurrent infectious complications, thus allowing patients to receive adequate systemic treatment for lymphoma given in a timely manner, resulting in improvements in complete remission and survival...."
NEW YORK DEC 27, 2005 (Reuters Health) - With the advent of highly active antiretroviral therapy (HAART), the overall survival of patients with HIV-related diffuse large-cell carcinoma is approaching that of patients without HIV infection, according to researchers.
"In assessing those factors that predict for better survival of diffuse large-cell carcinoma patients in the HAART era, we found that the standard prognostic factors in HIV-negative patients are applicable to patients with AIDS-related diffuse large-cell carcinoma as well," senior investigator Dr. Alexandra M. Levine told Reuters Health.
Factors that predict shorter survival, she added, "include older age, elevated LDH levels in blood, stage III or IV disease, poor performance status of the patient, and presence of two or more extra-nodal sites of lymphoma at diagnosis."
In the November 20th issue of the Journal of Clinical Oncology,
Dr. Levine of the University of Southern California, Los Angeles and colleagues retrospectively compared outcomes of 120 HIV- infected patients with diffuse large-cell carcinoma who did not receive HAART with those of 72 patients who were diagnosed later and did receive HAART.
There were no significant differences in HIV or diffuse large-cell carcinoma characteristics during these time periods, but the complete response rate rose from 32% pre-HAART to 57% in the HAART era (p = 0.0006). Median survival rose from 8.3 to 43.2 months (p = 0.0005).
In the pre-HAART era, those at high risk, according to the International Prognostic Index, had a 5 percent 3-year survival. For those at low-intermediate risk, survival was 20% and for low-risk patients, it was 22%.
However, using the International Prognostic Index to classify HAART patients yielded corresponding survivals of 64%, 64% and 50%. In addition, CD4 counts of less than 100 cells per microliter predicted shorter survival only in the pre-HAART era.
Given these improvements, the researchers conclude that "it is important to study the results of newer treatment paradigms and strategies that are currently used in patients with de novo diffuse large-cell carcinoma in patients with HIV-related diffuse large-cell carcinoma as well."
SOURCES: J Clin Oncol 2005;23:8477-8482.
Prognostic Factors in HIV-Related Diffuse Large-Cell Lymphoma: Before Versus After Highly Active Antiretroviral Therapy
Journal of Clinical Oncology, Vol 23, No 33 (November 20), 2005: pp. 8477-8482
Soon-Thye Lim, Roksana Karim, Anil Tulpule, Bharat N. Nathwani, Alexandra M. Levine
From the Division of Hematology, Department of Medicine; Division of Biostatistics, Department of Preventative Medicine; and Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; Department of Medical Oncology, National Cancer Center, Singapore, Singapore
Address reprint requests to Alexandra M. Levine, MD, University of Southern California, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail: alevine@usc.edu
ABSTRACT
PURPOSE: To compare the prognostic factors for survival and the validity of the International Prognostic Index (IPI) in patients with HIV-related diffuse large-cell lymphoma (HIV-DLCL) treated with curative intent in the pre-highly active antiretroviral therapy (HAART) era versus the HAART era.
PATIENTS AND METHODS: We retrospectively reviewed 192 patients with HIV-DLCL diagnosed from 1982 to 2003. Pre-HAART era included 120 patients who did not receive HAART, whereas the HAART era included 72 patients diagnosed after January 1997 who received HAART.
RESULTS: There were no statistically significant differences in terms of either lymphoma or HIV-related characteristics in the two time periods. The complete response rate improved from 32% in the pre-HAART to 57% in the HAART era (P = .0006), and median survival time improved from 8.3 to 43.2 months (P = .0005). In groups with low-, low-intermediate-, and high-intermediate-risk IPI disease, 3-year overall survival rates were 20%, 22%, and 5% in the pre-HAART era and 64%, 64%, and 50% in the HAART era, respectively. On multivariate analysis, factors independently associated with decreased survival in both periods were increasing IPI scores and failure to attain complete remission, whereas CD4 less than 100 cells/mL predicted shorter survival in only the pre-HAART era.
CONCLUSION: Prognostic factors and overall survival of patients with HIV-DLCC have changed. Clinical outcomes in patients with HIV-DLCL are now approaching the outcomes of patients with de novo lymphoma.
INTRODUCTION
In the era before the widespread use of highly active antiretroviral therapy (HAART), treatment of patients with AIDS-related lymphoma (ARL) using either standard or reduced doses of combination chemotherapy was associated with poor survival outcome.1,2 Thus, in the trial conducted by the AIDS Clinical Trials Group comparing standard-dose methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (M-BACOD) with reduced-dose M-BACOD, the median survival times of patients with systemic ARL receiving full-dose and reduced-dose chemotherapy were only 6.8 and 7.7 months, respectively.1 Poor prognostic factors for survival in patients with systemic ARL in the pre-HAART era included a Karnofsky performance status (KPS) of less than 70%, a history of AIDS before lymphoma, a CD4 cell count less than 100 cells/mL, stage III or IV disease, elevated lactate dehydrogenase (LDH) level, a history of injection drug use, and age 35 years or older.2-5 Pathologic subtype was not found to be of prognostic significance in these earlier studies. Thus, treated similarly, the prognosis of patients with all aggressive subtypes was equally poor.1
The use of HAART has led to a dramatic decline in AIDS-related illnesses and a remarkable improvement in overall survival among patients with AIDS.6 Likewise, there has also been significant improvement in the prognosis of patients with ARL, such that the median overall survival of these patients now approaches that of patients with de novo aggressive non-Hodgkin's lymphoma (NHL).7-12 With these changes in survival, it is highly likely that prognostic factors in patients with systemic ARL may have changed as well. Furthermore, in the HAART era, several studies have recently suggested the importance of pathologic subtype in terms of prognosis and survival. Thus, in a recent study of 322 patients with systemic ARL treated with curative intent, the survival time of patients with diffuse large-cell lymphoma (DLCL) in the HAART era was 43.2 months, compared with 5.7 months in patients with Burkitt's lymphoma.13 The significance of pathologic subtype was maintained on multivariate analysis.13 Similarly, in another study involving 74 patients with systemic ARL treated with infusional rituximab, cyclophosphamide, doxorubicin, and etoposide together with HAART, the pathologic subtype of Burkitt's lymphoma was also found to be an independent poor prognostic factor for survival.14 Thus, in the HAART era, considering Burkitt's lymphoma and DLCL as separate entities may provide more valid information when considering prognostic factors associated with survival.
The International Prognostic Index (IPI) was originally developed based on the clinical characteristics of patients with lymphoma subtypes corresponding to the International Working Formulation categories F, G, and H.15 Patients with Burkitt's lymphoma were not included in the model. Additionally, the IPI included only those patients who had received doxorubicin-containing combination chemotherapy. In patients with ARL, several studies have evaluated the usefulness of the IPI in predicting survival.5,16 However, these studies have generally included patients with all pathologic subtypes, including Burkitt's lymphoma, and have also included patients who did not receive treatment with curative intent for their lymphoma.5,16
The aim of this retrospective review was to evaluate the prognostic factors for survival in HIV-infected patients with DLCL in both the pre-HAART and HAART eras and to determine whether these factors have changed with the use of HAART. We also wished to compare the validity of the IPI in both the pre-HAART and HAART eras. Only patients with DLCL who had received combination chemotherapy with curative intent were included in an attempt to remove potential biases that might otherwise complicate the analysis.
PATIENTS AND METHODS
This study was approved by our institutional review board. The diagnostic criteria used to determine lymphoma subtype were based on the Lukes and Collins classification system for malignant lymphomas.17 Thus, for this study, the Lukes and Collins diffuse large cleaved, large noncleaved, and B immunoblastic subtypes were considered as DLCL. Using these diagnostic criteria, we retrospectively identified 228 patients with biopsy-confirmed DLCL who were seen at Los Angeles County-University of Southern California Medical Center and University of Southern California/Norris Cancer Hospital between 1982 and December 2003. All patients in this study were reviewed and diagnosed by a single, expert hematopathologist (B.N.N.).
Among the 228 patients, 192 received combination chemotherapy with curative intent and were included in this study. Patients who did not receive curative therapy were excluded. All patients were HIV seropositive by enzyme-linked immunosorbent assay with confirmation by Western blot.
All patients were staged before treatment using the Ann Arbor staging system, which included a history; physical examination; standard laboratory tests; computed tomography scans of chest, abdomen, pelvis and other relevant sites; gallium scans; and bone marrow biopsy. Lumbar puncture was routinely performed in all patients, and assessment of CSF cytology was also performed in all patients.
In this study, patients in the pre-HAART era included patients who were diagnosed before December 31, 1996, and who did not receive HAART therapy. Patients in the HAART era included patients who were diagnosed after January 1, 1997, and who did receive HAART, including both patients who were already on HAART before a diagnosis of lymphoma as well as patients who were started on HAART after a diagnosis of lymphoma. It has been our center's practice to administer HAART concurrent with combination chemotherapy.
The following HIV-related and lymphoma-related characteristics were assessed: age, sex, use of injection drugs, CD4 count at presentation, prior history of AIDS, IPI score, and response to treatment. Differences in baseline lymphoma characteristics and HIV-related characteristics between the patients treated in the HAART and pre-HAART eras were compared using the t test or 2 test for continuous or categoric variables, respectively. Survival and follow-up time were calculated from the date of diagnosis to death or to the date when the patient was last seen, respectively. Comparisons were made for pre-HAART and HAART eras. Three-year survival rates were compared between the two time periods stratified by IPI risk categories. The Cox proportional hazards model was fitted to determine the significant prognostic factors for survival. Factors showing significant impact in univariate analysis were tested in a multivariate model for independence of association. Survival curves were compared between the pre-HAART and HAART eras using Kaplan-Meier survival analysis.18 P values from the log-rank tests were reported for differences in median survival time.18 All analyses were performed using SAS version 8.0 (SAS Institute, Cary, NC).
DISCUSSION
The prognosis of HIV-infected patients with DLCL has improved dramatically in the HAART era. It is conceivable that better immune reconstitution through the use of HAART has reduced the risk of serious intercurrent infectious complications, thus allowing patients to receive adequate systemic treatment for lymphoma given in a timely manner, resulting in improvements in complete remission and survival. Although not statistically significant (P = .08), the median CD4 cell counts have increased from 70 cells/mL in the pre-HAART era to 94 cells/mL in the HAART-treated patients. Along with this significant improvement in survival, we found that factors predictive of prognosis have changed. Although both HIV-related (CD4 cell count) and lymphoma-related (attainment of complete remission and IPI score) factors were independently predictive of survival in the pre-HAART era, only lymphoma-related factors were independently prognostic for survival in the HAART era. Furthermore, the IPI score was more predictive for survival in the HAART era compared with the pre-HAART era (P = .01).
Although the majority of patients with DLCL presented with advanced stages of disease and elevated LDH, almost three quarters of patients in both time periods still fell within the IPI low-risk and low-intermediate-risk groups. This pattern of patient distribution among IPI risk groups is comparable to that of HIV-uninfected patients, of whom almost two thirds also present with low- or low-intermediate-risk IPI disease.19 However, this observation is in contrast to the findings from a previous study by Rossi et al.5 In that study, which evaluated the utility of the age-adjusted IPI score in predicting for survival in 69 patients with systemic ARL, only 22% of patients were classified in the low- or low-intermediate-risk IPI groups. However, the study included a large number of patients with either small noncleaved-cell lymphoma or unspecified high-grade lymphoma (48%), both of which were excluded in the original IPI analyses.19 The exclusion of patients with Burkitt's lymphoma in our current study may have accounted for these differences in the pattern of patient distribution among the IPI risk groups. However, these differences could also be a result of selection bias resulting from the exclusion of patients who did not receive curative treatment for their lymphoma (n = 32, 16%).
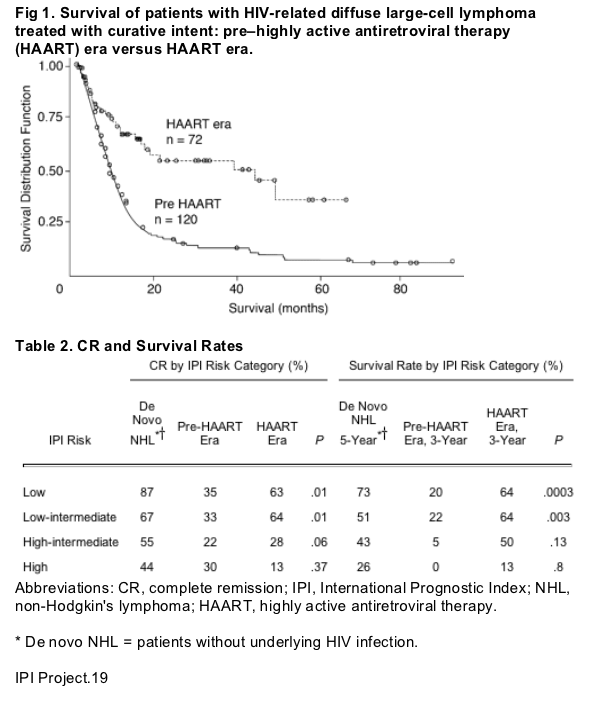
One of the objectives in developing the IPI was to enable the identification of specific risk groups of patients with de novo NHL so that selected therapeutic approaches could be applied.19 Although the IPI was also found to be predictive of survival in patients with ARL in the pre-HAART era,5 its usefulness in identifying subgroups of patients for specific treatment approaches was quite limited because survival of patients in all risk categories was poor, with an overall 3-year survival rate of only 20% even among patients within the lowest IPI risk category. Before the use of HAART, these survival rates were considerably inferior to the rates in patients with de novo NHL who were in a similar risk group category (Table 2).
In contrast, in the HAART era, we found that the 3-year overall survival rates of patients with HIV-DLCL in the low-risk (64%), intermediate-low-risk (64%), and high-intermediate-risk (50%) categories after treatment with CHOP- or M-BACOD-based chemotherapy seemed to approach the corresponding 5-year survival rates (Table 2) of patients with de novo NHL treated with doxorubicin-containing regimens.19 Of note, although the survival of HIV-infected patients with high-intermediate-risk IPI was comparable to the survival of patients with de novo NHL, the complete remission rate was considerably worse (Table 2). However, the small number of patients (n = 9) in this risk category may limit direct comparisons. Overall, similar to patients with de novo NHL, the prognosis of HIV-infected patients with DLCL in the HAART era worsened with increasing IPI scores, although the prognosis of HIV-related patients with high-risk IPI disease seemed particularly poor in this study, with a 3-year survival rate of only 13%.
Importantly, it should be noted that the median CD4 count among the group of HIV patients with high-risk IPI disease was only 60 cells/mL, which was considerably lower than the median CD4 counts of patients in the other IPI risk groups. The current observation that patients with the lowest median CD4 counts presented with IPI high-risk disease is also of interest. This observation could be explained by the fact that patients with profound immunodeficiency are more likely to have poor performance status, which is one of the components of the IPI scoring system. However, it is also conceivable that a severely immunodeficient host may less effectively control the underlying malignancy, thereby resulting in more extensive lymphomatous disease. Nonetheless, the precise relationship between the level of immunosuppression and IPI score remains to be fully elucidated.
The poor outcome of patients with high-risk IPI HIV-DLCL after receipt of standard chemotherapy is similar to that demonstrated in HIV-uninfected patients with NHL, in whom the 5-year survival rates among patients with IPI intermediate-risk and IPI high-risk disease were only 43% and 26%, respectively.19 To improve the outcomes of patients with advanced de novo diffuse large B-cell lymphoma, strategies, such as use of monoclonal antibody therapy in combination with chemotherapy,20 increasing dose-intensity,21 and/or upfront transplantation,22,23 have been explored. It may now be important to also investigate the role of these strategies in patients with HIV-DLCL. Currently, encouraging results have been reported in recent trials evaluating novel regimens, such as infusional EPOCH and rituximab, cyclophosphamide, doxorubicin, and etoposide, in patients with systemic ARL.11,14 In the former study,11 it is interesting to note that the survival rate at 53 months among patients with high-intermediate-and high-risk IPI disease was an encouraging 44%, suggesting that the EPOCH regimen may be particularly useful in these subsets of patients.
Recently, we and others have also shown that the survival of patients with HIV-related Burkitt's lymphoma in the HAART era is significantly inferior to the survival of patients with HIV-DLCL after receipt of standard CHOP-like chemotherapy, strongly suggesting that histology is now an important consideration in the treatment of patients with ARL.13,14 Furthermore, our current work and data from Spina et al14 also suggest that HIV-related factors are becoming less important, given the availability of HAART. Taken together, these data support the concept that lymphoma-related characteristics are now more important considerations than HIV-related factors in the management of ARL in the era of HAART. Currently, several studies have stratified patients with ARL into different therapeutic groups based on characteristics such as history of prior AIDS and CD4 counts.24 Such an approach may be less relevant now that HAART is routinely used. Instead, similar to NHL in HIV-uninfected patients, emphasis should be placed on lymphoma-specific characteristics, such as histology and IPI.
In conclusion, the use of HAART along with combination chemotherapy has resulted in overall survival times for patients with HIV-DLCL that approach the survival times of patients without underlying HIV infection. Therefore, it is important to study the results of newer treatment paradigms and strategies that are currently used in patients with de novo DLCL in patients with HIV-DLCL as well.
RESULTS
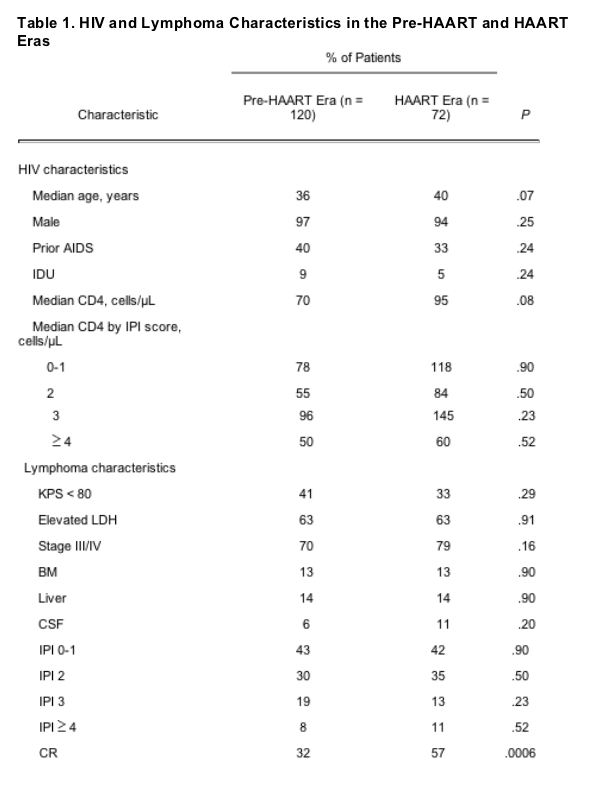
HIV and Lymphoma Characteristics
The presenting HIV- and lymphoma-related characteristics of the 192 patients with DLCL are listed in Table 1, including 120 patients diagnosed in the pre-HAART era and 72 patients diagnosed in the HAART era. There were no statistically significant differences between the two groups of patients at presentation in terms of age, sex, history of injection drug use, prior history of AIDS (opportunistic infection or Kaposi's sarcoma), LDH level, or stage of disease at diagnosis. There was also no difference between the two groups in terms of the distribution of patients by IPI risk category. Although the median CD4 count of patients with DLCL in the HAART era was higher than that of patients in the pre-HAART era, this difference was only of borderline statistical significance (95 v 70 cells/mL, respectively; P = .08). In the pre-HAART era, the median CD4 count by IPI risk category did not differ significantly across the various IPI risk groups, with median CD4 counts ranging from 50 to 96 cells/mL. In the HAART era, the median CD4 count of patients with IPI high-risk disease (60 cells/mL) was considerably lower than the CD4 counts of patients in the other IPI risk groups (Table 1).
Treatment
All patients in this study received combination therapy with curative intent. The most commonly used regimens were M-BACOD and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) -based chemotherapy, which were used in 91% and 95% of patients in the pre-HAART and HAART eras, respectively. Other regimens used in the HAART era included the infusional etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) regimen in three patients and infusional cyclophosphamide, doxorubicin, and etoposide in two patients. In the pre-HAART era, other regimens used included various combinations of high-dose cytarabine, doxorubicin, etoposide, vincristine, cyclophosphamide, methotrexate, and prednisolone. All patients with negative CSF cytology were treated with four weekly doses of prophylactic intrathecal cytarabine. Patients with positive CSF cytology were treated with intrathecal cytarabine on alternate days until clearance of malignant cells was achieved. Thereafter, the patients were treated with weekly intrathecal chemotherapy for a month, followed by monthly intrathecal therapy for a year.
Outcome
The complete remission rate among HIV-infected patients with DLCL improved from 32% in the pre-HAART era to 57% in the HAART era (P = .0006), whereas the overall median survival time improved from 8.3 to 43.2 months (P = .0005; Fig 1). Table 2 lists the complete remission rates and 3-year overall survival rates for patients treated in both the pre-HAART and HAART eras. As shown, within the groups of patients with low-risk (IPI score 1) and low-intermediate-risk (IPI score = 2) disease, the complete remission rates and 3-year overall survival rates were significantly higher for patients treated in the HAART era compared with patients treated in the earlier time period. Although the complete remission rate and overall median survival time for patients with high-intermediate-risk disease (IPI score = 3) in the HAART era were higher than those seen in the pre-HAART era, these differences did not reach statistical significance. High-risk DLCL (IPI score 4) was associated with low rates of complete remission and short survival, with no differences between the pre-HAART and HAART eras.
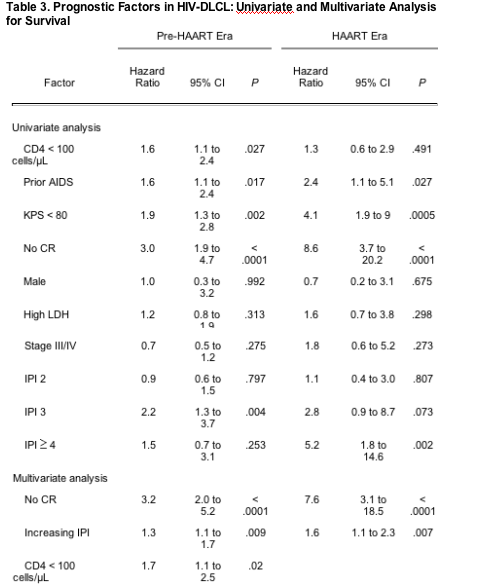
In the univariate analyses of poor prognostic factors for survival in the pre-HAART era, KPS less than 80, a prior history of AIDS, a median CD4 count less than 100 cells/mL at diagnosis, and failure to attain complete remission were each associated with decreased survival (Table 3). High-intermediate-risk IPI score was also associated with decreased survival. On multivariate analyses, failure to attain complete remission, a CD4 count less than 100 cells/mL, and an increasing IPI score were independently predictive of decreased survival (Table 3). In the univariate analyses of poor prognostic factors for survival in the HAART era, KPS less than 80, a prior history of AIDS, failure to attain complete remission of disease, and a high-risk IPI score were each associated with decreased survival. An intermediate-high-risk IPI score was also associated with decreased survival, although the association was only of borderline significance (P = .07). Of importance, CD4 count less than 100 cells/mL was no longer associated with an inferior survival outcome. On multivariate analysis, only an increasing IPI score (P = .007) and failure to attain complete remission (P < .0001) were independently associated with decreased survival. Although an increasing IPI score was associated with an inferior survival outcome in both the pre-HAART and HAART eras, IPI score was more predictive of survival in the HAART era compared with the pre-HAART era (hazard ratio = 1.6; 95% CI, 1.1 to 2.3 in the HAART era; and hazard ratio = 1.3; 95% CI, 1.1 to 1.7 in the pre-HAART era; P = .01 for interaction).
Abbreviations: HIV-DLCL, HIV-related diffuse large-cell lymphoma; HAART, highly active antiretroviral therapy; KPS, Karnofsky performance status; CR, complete remission; LDH, lactate dehydrogenase; IPI, International Prognostic Index.
|
|
| |
| |
|
|
|