 |
 |
 |
| |
Improving Lipoatrophy:
Uridine and Pravastatin studies raise hope of new interventions to combat fat loss
|
| |
| |
Lipodystrophy Workshop
Exclusive report for NATAP
November 16, 2005
Dr Graeme Moyle
Chelsea and Westminster Hospital, London
Introduction and Background
This summary will cover key posters and presentations at the 5th Adverse drug events and Lipodystrophy workshop. The report will largely look at clinical data potentially of practical applicability. The focus of the summary will be fat loss (lipoatrophy).
The development of clinical lipoatrophy is a highly stigmatising adverse effect associated with long-term antiretroviral therapy. A number of risk factors for the development of lipoatrophy have been identified including drug choice, factors related to HIV disease and immune function and host genetics. The only modifiable factor identified is with regards to choice of antiretroviral agent although, arguably, earlier initiation of antiretroviral therapy may also reduce relative risk. The choice of nucleoside analog and the use of at least some protease inhibitors appears to accelerate the loss of peripheral fat. Amongst the nucleoside analogs stavudine appears from prospective clinical trials data to be associated with a higher relative risk of clinical lipoatrophy and a more rapid limb fat loss as compared with zidovudine, abacavir, or tenofovir. Zidovudine (AZT) also appears a risk factor for lipoatrophy. Long term use of abacavir or tenofovir with 3TC or FTC do not appear to be risk factors for lipoatrophy.
Lipoatrophy may be assessed in a number of ways. Clinically it is characterised by loss of subcutaneous fat which becomes most evident in the face, the arms and legs leading to increased vein prominence and in the buttock area. The development of clinically evident lipoatrophy represents the “end stage” of a continuum. Studies recruiting individuals with moderate to severe clinical lipoatrophy generally find these individuals have limb fat mass approximately 50% below the limb fat mass of control or “normal” individuals (normal limb fat mass is generally 8kg or more). More objective evaluation of body composition for the purposes of assessing changes in limb fat over time includes anthropometry and DEXA scanning. The point at which individuals have clinically evident lipoatrophy is likely to vary based on individuals normal fat distribution (determined by such things as sex and ethnicity as well as family genetics) as well as changes in fat mass that have already occurred as a result of HIV infection (such as wasting).
A number of hypotheses have been put forward regarding the aetiology of lipoatrophy. Studies have reported associations, which may or may not be causal in nature, with changes in mitochondrial DNA or mitochondrial function, changes in expression of various adipocyte genes, or local levels of various inflammatory cytokines such as tumour necrosis factor-alpha. Animal models and in vitro systems for other (mainly genetic) forms of lipoatrophy exists but are not fully applicable to the setting of HIV and antiretroviral associated lipoatrophy. Furthermore, no reliable predictive tests or markers are currently established.
The only approach to its management of lipoatrophy that has so far demonstrated benefit in randomized trials is switching therapy from a thymidine analog to abacavir, tenofovir or a regimen which does not include NRTI drugs.
Predicting Lipoatrophy and its resolution
While a number of factors associated with an increased relative risk of developing lipoatrophy during treatment have been reported, changes in specific markers with treatment which may proceed and be a harbinger of future lipoatrophy have not been established. Using data from the ACTG384 study baseline and early (week 8) changes in clinical parameters after initiation of therapy were correlated with a decline in limb fat and > 20 percent from baseline to week 64. In individuals randomised to receive d4T plus ddI, baseline factors associated with greater fat loss in a univariate model included higher at baseline CD4, baseline body mass index, cholesterol and triglycerides. At week 8, greater increases in triglycerides and cholesterol were associated with greater fat loss. In a multivariate model, an increase in triglycerides at week 8 of 100 mg/dl was associated with a 3.27 increase in risk of losing > 20 percent fat from baseline. Assignment to d4T/ddI, relative to AZT/3TC increased the relative risk of 20% fat loss by 3.19 fold (47% vs. 17%, respectively). The association between early triglycerides change and fat loss was not observed in persons who received AZT/3TC. [Parker RA, et al]. As the combination of d4T/ddI is now infrequently used in clinical practice, regimens based on AZT may be the most common cause of incident lipoatrophy. Unfortunately, the data did not provide assistance in predicting who will develop this adverse event during AZT based treatment but did go to further underline that fat loss is associated with longer term use of this NRTI.
The other side of this story is whether predictive factors for the recovery of limb fat can be established. Investigators from Australia, using data derived from the MITOX (abacavir vs. thymidine NRTI) switch study and the ROSEY (rosiglitazone vs. placebo) intervention study evaluated factors associated with limb fat increase. The only baseline parameter significantly correlated with a greater than 0.5 kg increase was higher baseline body mass index (OR 1.6). Increases in visceral adipose tissue was noted to have a weak association with limb fat recovery. When considering decreases in visceral adipose tissue, independent baseline factors included higher baseline limb fat mass (that is, persons with higher baseline limb fat lost more visceral fat), lower HDL and high insulin as well as a greater decrease in limb fat at week 72. Increasing subcutaneous abdominal fat was also correlated with recovery in limb fat [Wand H, et al].
Data evaluating factors associated with limb that recovery during the RAVE study were reported as part of the main EACS conference. Data were derived from this randomized, 48-week study of change in limb fat after replacement of AZT or d4T with ABC or TDF in adults on HAART with moderate/severe lipoatrophy. Limb fat was measured by dual-energy x-ray absorptiometry (DEXA). Factors considered were treatment group, age, sex, risk group, ethnicity, baseline CD4, nadir CD4, CDC status, baseline limb fat, baseline thymidine analog, the number previously antiretrovirals, time on therapy, baseline weight and use of PI or NNRTI as third agent. A second analysis evaluated these factors with regards to recovery of >710g of limb fat, the upper quartile of fat recovery.
The study involved 105 adults receiving d4T (n=71) or AZT (n=34) who were randomized, 53 to ABC, 52 to TDF as replacement agents for the thymidine analog. The primary outcome of this study, reported at the CROI Retrovirus conference 2005, indicated that both abacavir and tenofovir replacement were similarly effective at allowing that recovery to occur. In looking at factors associated with that recovery univariable and multivariable models were built. In univariable regression analyses only age at randomisation (p=0.02) and baseline thymidine analogue (p=0.008) were associated with the degree of limb fat mass change over 48 weeks. A multivariable linear regression analysis found these factors were independently associated with change in limb fat mass at 48 weeks. Those who switched from AZT had mean increases in limb fat that were 419g lower, on average, than those switching from d4T. In a second analysis, focusing on those patients who recovered >710g of limb fat (the highest quartile (25%) in the study), the multivariable model found AZT use, longer duration of ARVs and white ethnicity to be associated with less likelihood of fat recovery. These data raise concern that lipoatrophy which occurs during AZT therapy may be relatively less reversible than that observed during d4T therapy. While available data suggest that the onset of clinically evident lipoatrophy with AZT may be less common and less severe than that observed with d4T, these data suggest that switching away from AZT prior to the development of clinical lipoatrophy would be prudent Moyle G, et al EACS].
Treating Lipoatrophy
In a substudy of RAVE, involving 47 individuals (23 switched to tenofovir, 24 to abacavir), a laser imaging technique was used to estimate facial volume changes, in order to assess restoration of fat in the face. 48 weeks after switch, laser imaging data indicated stabilisation or improvements in cheek volume in 68% of participants. Furthermore, over 30% of participants reported that their facial fat loss had improved. Increases in facial volume were similar in those switched to abacavir or tenofovir. The authors suggested that the mean volume increase in cheek fat was similar to the increase in cheek volume seen in a previous study that evaluated collagen injections in order to improve facial lipoatrophy. Of note, the changes in facial fat correlated with improvements in limb fat as measured by DEXA scan (p=0.02) suggesting that recovery of fat mass was occurring at all subcutaneous fat sites.
Beyond the RAVE study data, information from 3 small studies have pointed to potential new management approaches for lipoatrophy.
The first of the studies evaluated ddI as a replacement agent for d4T in a cohort of 27 Thai patients currently receiving d4T based regimens. The study included 15 women and 12 men. The average duration of prior d4T use was 17.2 months. 48 weeks after switching the mean bodyweight had increased from 54 kg to 58.2 kg. 25 of 27 individuals self-reported improvements in their lipoatrophy [Bowonwatanuwong C et al.]. This study due to funding and technological constraints did not use of objective assessments of lipoatrophy but suggest that ddI as a thymidine substitute is in circumstances where abacavir or tenofovir are not suitable options. The data are in keeping with previous studies that suggest the key issue in managing lipoatrophy is the removal of the thymidine analog from the treatment regimen.
HMG-Co-A reductase inhibitors (statins) are predominantly used to manage hypercholesterolemia but have a range of effects beyond cholesterol reductions, which contribute to the benefits of these agents with regard to reducing the risk of cardiovascular disease. In a randomised study involving 33 hypercholesterolaemic men, 16 receiving pravastatin 40 mg QD, 17 placebo, pravastatin was observed to significantly reduce cholesterol exposure (as measured by area under the curve (AUC)) relative to placebo. The effect observed was similar to that reported in previous published studies. The surprise observation in this study was that limb fat was observed to increase over the 12 weeks of treatment. Individuals in the pravastatin group experienced an increase in total fat, by DEXA scan, of 1.03 kg wear as placebo recipients experienced a small decline of 0.09 kg (p = 0.01). The critical parameter of limb fat increased significantly more in those individuals who received pravastatin, 0.72 kg as compared with the placebo recipients, 0.19 kg (p = 0.04). Of further interest was the observation that pravastatin recipients showed a trend towards lower intra abdominal fat as measured by CT scan although the change observed was only a 2.9% decline [Mallon PWG et al].
The most exciting of the three studies looking at interventions for lipoatrophy involved supplementation with a dietary supplement containing substantial amounts of the nucleoside base uridine. In vitro, uridine has been reported to protect a range of different cell lines from mitochondrial toxicity associated with particular nucleoside analog drugs. A small study conducted in Finland involving 20 individuals randomised individuals with clinical lipoatrophy to either receive the commercial uridine supplement NucleomaxX or placebo. NucleomaxX is dosed three times a day and taken for ten days out of every month (36 grams NucleomaxX three times a day) it has been documented to increase plasma uridine levels. One patient discontinued the study due to the poor taste of the supplement. All patients were receiving a thymidine analog based regimen and had clinically evident lipoatrophy. Details of baseline characteristics and changes in these parameters over three months of therapy are shown in the table. Changes in limb fat were measured using proton spectroscopy, a technique for assessing body composition which has not been previously widely used. As detailed in the table, individuals who received the supplement observed substantial and significantly greater increases in limb than those in the placebo group. The increase in limb that reported, approximately 900 g in three months is substantially greater and more rapid than that reported from studies such as MITOX or RAVE, where a thymidine analog was replaced by a non-thymidine. No important adverse events were reported during use of the supplement [Sutinen J et al].
Table 1:
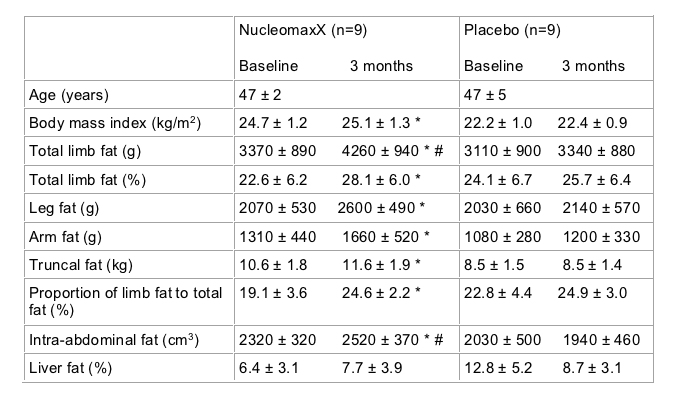
A second study involving 16 individuals with lipoatrophy currently receiving a stavudine based regimen reported the use of the same uridine supplement over 16 weeks followed by a further period of follow-up off therapy of 16 weeks. The study did not involve a control population making the data more difficult to interpret. Fourteen patients completed the study with no changes in ART regimens during the study. No adverse events related to treatment were reported. Body mass index did not change during the course of treatment with the uridine supplement. However, patients and physician assessment of lipoatrophy improved from baseline to week 16 with further benefit observed in the 16 weeks following the discontinuation of uridine supplementation. As this was an open label study the interpretation of self-reported and position reported data are challenging. No objective assessments of limb or body fat, such as anthropometry, DEXA or CT were reported. No significant changes in disease markers, liver function tests, lipids, insulin sensitivity, or lactate were observed. A non-significant trend for an increase in mitochondrial DNA content in and the sights on fat biopsy was observed although no substantial or significant changes in PBMC mitochondrial DNA content was observed [McComsey G, et al].
Taken together these two studies provide the suggestion that uridine supplements maybe a new treatment tool in managing lipoatrophy. There use beyond persons receiving pyrimidine NRTIs (and specifically thymidine analogs) has not been evaluated. Furthermore, it is important to note that the studies reported involved small numbers of individuals and did not use endpoints that could be considered standard or are part of the established lipodystrophy case definition approach. In particular, self-report of lipoatrophy is widely thought by physicians working in this area to be of limited value. Larger placebo-controlled studies using established objective endpoints such as DEXA scanning are now required. In addition, limited safety data have been reported with this supplement and details of possible drug interactions with life-saving antiretroviral medications have not been reported. A number of supplements, notably garlic supplements and St John’s wort, are known to have important interactions with some HIV medications. Before individuals rush to buy expensive uridine supplements over the Internet, they should consider that there are potential risks which have not been evaluated and benefits which have not been established to standards that would be normally used by regulatory agencies.
|
|
| |
| |
|
 |
 |
|
|