 |
 |
 |
| |
Tipranavir & Effect of Using Active HIV Drugs
|
| |
| |
Reported by Jules Levin
"Tipranavir/ritonavir (TPV/r) Demonstrates Superior Treatment Response to Lopinavir/r (LPV/r), Amprenavir/r (APV/r), and Saquinavir/r (SQV/r) in PI-experienced Patients From the TPV RESIST-1 and RESIST-2 Trials"
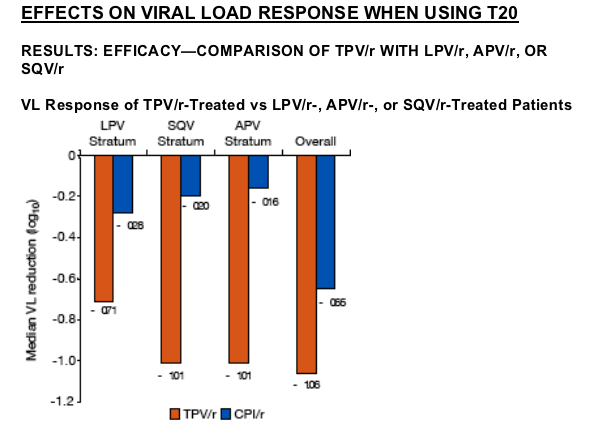
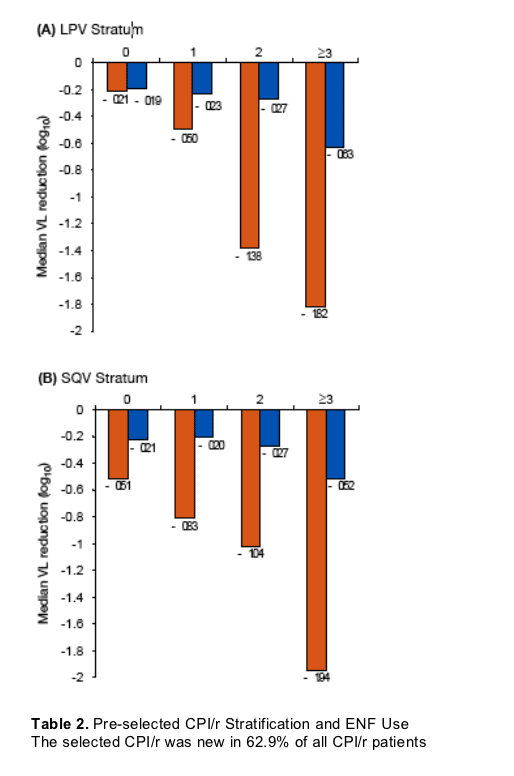
The study analysis on RESIST 1 & 2 reported below discusses the antiviral effect of tipranavir in highly treatment experienced patients in the RESIST studies, and the effect of adding 0, 1, 2 or 3 active drugs along with tipranavir. As well, the analysis reports the effect of adding T20. T20 is a fusion inhibitor, a new class of HIV drugs, and therefore is a potent treatment option and patients will not have pre-existing resistance since it's a new HIV drug class. Adding T20 had the effect of doubling the viral load reduction from about -1 log to -2 logs HIV RNA decline. This analysis also shows below that when 0 new active drugs were used besides tipranavir viral load was mostly still reduced compared to the comparator PI arm, but with the addition of 1, 2, or 3 active the viral load reductions increased more each time another active drug was used. Of course, using 3 active drugs resulted in the greatest reductions in viral load from -1.50 to -1.97 logs. Additionally, the tipranavir treated subjects consistently had better viral load reductions than the comparator PI arms regardless of whether additional drugs were used.
The 3rd IAS Conference (IAS 2005)-Rio
Poster WePe6.3C07
Lazzarin A,1 Mukwaya G,2 Clumek N,3 Workman C,4 Gathe J,5 Trottier B,6 Villacian J,2 Neubacher D,2 McCallister S2
1. Fondazione Centro San Raffaele del Monte Tabor, Milan, Italy; 2. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA;
3. Hopital Universitaire St Pierre, Brussels, Belgium; 4. AIDS Research Initiative, Darlinghurst, Australia;
5. Therapeutic Concepts, Houston, TX, USA; 6. Clinique Medicale l'Actuel, Montreal, Canada
Tipranavir (TPV) is a non-peptidic protease inhibitor (NPPI) that was recently approved by the US Food and Drug Administration. The companion phase 3 RESIST-1 and RESIST-2 trials demonstrated that TPV/r was superior to a standard-of-care boosted PI at 24 weeks. TPV has a resistance profile distinct
from currently available PIs and retains broad and potent in vitro antiviral activity against a wide range of patient-derived HIV isolates resistant to peptidic PIs. Furthermore, TPV-based therapy produces potent, durable, and tolerable therapy in treatment-naive and PI-experienced HIV-1 infected patients. The trials were designed to allow combination of data from RESIST-1 and RESIST-2.
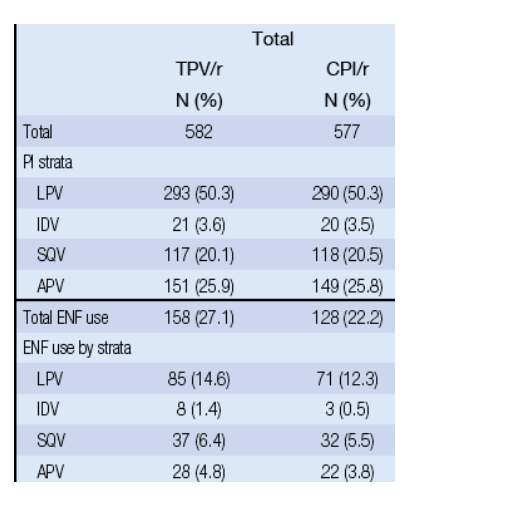
The planned analyses presented here compare the safety and efficacy of TPV/r vs LPV/r (the most frequently prescribed CPI across the 2 trials), APV/r, and SQV/r, and the effect of including active antiretrovirals, including genotypically active agents (drugs in OBR that show genotypic sensitivity) and enfuvirtide (ENF), in the background regimen.
AUTHOR SUMMARY
_TPV/r demonstrated superior virologic response vs LPV/r, SQV/r, and APV/r
(P<0.0001)
The efficacy of both TPV/r and CPI/r was progressively enhanced by the number of active drugs in the background regimen
— TPV/r was always superior to LPV/r, SQV/r, and APV/r regardless of the
number of background ARVs
Addition of ENF, an active background drug, resulted in superior virologic
response with TPV/r than LPV/r, SQV/r, and APV/r
TPV/r provides a potent option for management of treatment-experienced
Patients
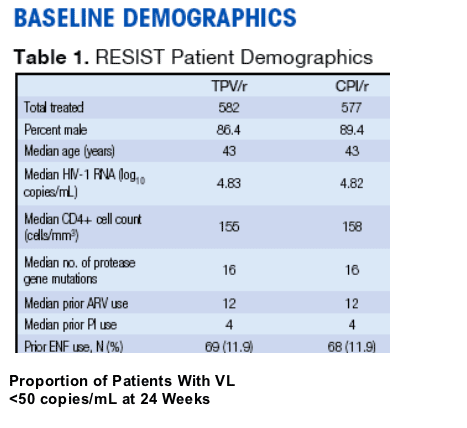
Proportion of Patients With VL
<50 copies/mL at 24 Weeks
_In addition, 34.2% of TPV/r patients had VL <400 copies/mL
compared with 14.9% of CPI/r patients
— LPV stratum: TPV/r 34.1% vs LPV/r 18.3%
— SQV stratum: TPV/r 34.2% vs SQV 9.3%
— APV stratum: TPV/r 34.4% vs APV 14.1%
ABSTRACT
OBJECTIVES: The RESIST trials are phase 3, prospective, multicenter, randomized, open-label studies demonstrating the statistical superiority of TPV/r over optimized standard of care ritonavir-boosted PIs. The objective of this planned analysis was to compare the efficacy of TPV/r to that of LPV/r, APV/r, IDV/r, and SQV/r.
METHODS: Patients with >3-class or more antiretroviral experience
including >2 or more PI-based regimens; >1 or more primary PI mutation and
2 or less at amino acids 33, 82, 84, 90; and viral load (VL) >1000 copies/mL and any CD4+ cell count were eligible. Before randomization, an optimized CPI/r-based regimen was selected; the selected PI could be new or from the current regimen.
RESULTS: 1483 patients with a median baseline VL of 4.8 log10 copies/mL, and median CD4+ cell count of 162 cells/mm3 were treated. Pre-selected CPIs were LPV (50%), APV (26%), SQV (20%), or IDV (4%).
The median VL response at 24 weeks with TPV/r and CPI/r, respectively, was -0.71 and -0.28 log10 in the LPV stratum, -1.01 and -0.20 log10 in the SQV
stratum, and -1.01 and -0.16 log10 in the APV stratum.
The VL response with TPV/r in these strata was -1.79 to -2.20 log10 when enfuvirtide was included in the background regimen compared to -0.56 to -0.63 log10 without enfuvirtide.
By contrast, the median VL response in the CPI strata with enfuvirtide was -0.28 to -0.43 log10 and without was -0.14 to -0.26 log10.
TPV/r was consistently associated with a greater response than CPI/r when combined with 0 (-0.02 to -0.72 log10), 1 (-0.27 to -0.63 log10), 2 (-0.77 to -1.13 log10), or >3 (-0.49 to -1.42 log10) active drugs.
CONCLUSION: TPV/r provides a statistically superior VL response compared with LPV/r, APV/r, and S
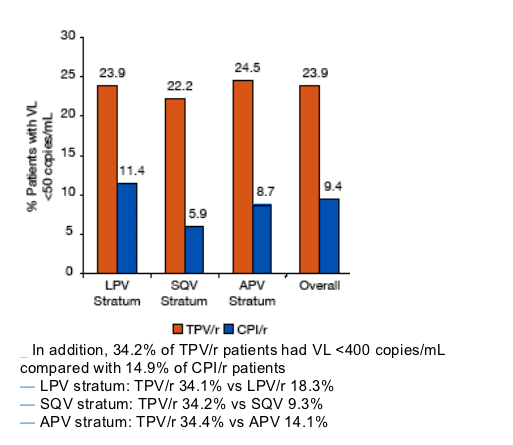
For the RESIST trials, treatment response (2 consecutive VL measurements >1 log10 below baseline) was observed in 41.2% of TPV/r patients compared with 18.9% of CPI/r patients (P<0.0001)
RESULTS: EFFECT OF ENF USE ON VIRAL LOAD RESPONSE
Figure 4. VL Response at 24 Weeks in PI Strata With ENF
|
| |
|
 |
 |
|
|