 |
 |
 |
| |
Overview of Phase 1 and 2a Safety and Efficacy Data of Maraviroc (UK-427,857)
|
| |
| |
Reported by jules Levin
3rd IAS Conference on HIV Pathogenesis and Treatment
Rio de Janeiro, Brazil 24th-27th July 2005
Mary McHale, Sam Abel, Deborah Russell, James Gallagher, Elna van der Ryst
Pfizer Global Research and Development, Sandwich Laboratories, Kent, UK.
This is poster presented at Rio.
SUMMARY & CONCLUSIONS
MVC was well-tolerated at doses up to and including 300 mg BID in this
population of healthy volunteers and asymptomatic HIV-positive patients.
- At doses of 300 mg BID or less, the AE profile was similar to that of
placebo, with only headache, nausea and flatulence occurring significantly
more frequently in patients receiving MVC compared to placebo.
- Postural hypotension was only seen at rates higher than placebo in MVC
patients for unit doses of ≥600 mg.
- Clinically-relevant elevations in transaminases occurred sporadically in all
groups (placebo- and MVC-treated), with no dose relationship and no
associated elevations in bilirubin.
- There was no evidence of clinically-relevant prolongation of QTc interval at
multiple doses of MVC ≦1200 mg QD.
- MVC doses ≥100 mg BID resulted in mean viral load reductions of
>1 log10 copies/mL, up to 1-6 to 1.84 log reductions (see tables immediately below)
- Dosing with food had no significant effect on viral load reduction.
- Dosing at 150 mg BID and 300 mg QD resulted in similar viral load
reductions.
MVC is currently in Phase 2b/3 development in both treatment-naive and
experienced patients using doses equivalent to 300 mg QD and 300 mg BID.
Efficacy data
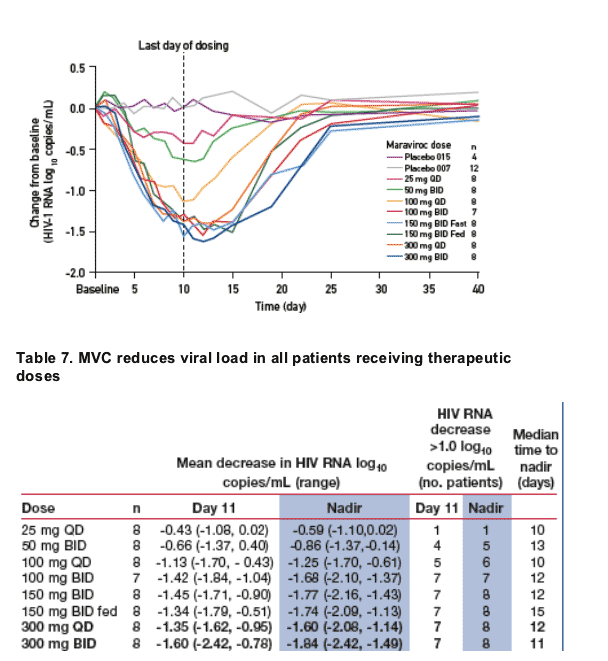
All doses of ≥100 mg BID showed similar viral load reductions with delayed
viral rebound and maximum viral load reductions occurring after
discontinuation of dosing (Figure 2 and Table 7).
Figure 2. Mean reduction in viral load over time
Emergence of CXCR4-Tropic Virus1,5
61/63 patients (97%) with CCR5-tropic virus at baseline remained
CCR5-tropic following ten days of MVC monotherapy.
Two patients showed emergence of CXCR4-using variants during treatment
with MVC 100 mg QD for ten days, with R5 variants remaining the
predominant virus species post-treatment:
Patient 1 - HIV RNA decline: -0.71 log10 copies/mL
- Transient emergence of dual/mixed-tropic virus at day 11
- Reverted to CCR5-tropic at day 40.
Patient 2 - HIV RNA decline: -1.26 log10 copies/mL
- Dual/mixed-tropic virus detected from day 11 onwards
- R5 variants constituted ~80% of the circulating virus after one
year's follow-up.
Phylogenetic analysis suggested that the R5/X4 variants which emerged posttreatment in each patient most likely expanded from a pre-treatment R5/X4
virus reservoir, rather than co-receptor switching of an R5 clone.
INTRODUCTION & OBJECTIVES
There is an urgent medical need for antiretroviral agents with novel
mechanisms of action in order to address the emergence of HIV resistant or
cross-resistant to current antiretroviral agents in HIV-1- infected patients.
Maraviroc (MVC; UK-427,857) is a CCR5 antagonist with potent anti-HIV
activity in vitro and in vivo against CCR5-tropic HIV-1 strains.
Because MVC targets an extracellular host target, it is active in vitro against
current drug class-resistant CCR5-tropic HIV strains.
An extensive Phase 1/2a and drug-drug interaction development
programme has been conducted, involving more than 600 healthy volunteers
and patients.
The development programme to date has demonstrated that MVC is
well-tolerated at doses up to and including 300 mg BID and that 10-day
monotherapy resulted in significant reductions in viral load.1-4
MVC is currently in Phase 2b/3 development in both treatment-naive and
experienced patients using doses equivalent to 300 mg QD and 300 mg BID.
The objective of this study was to evaluate and report the safety and efficacy
of MVC by combining data from six multiple-dose Phase 1/2a studies in
healthy volunteers and asymptomatic HIV-positive patients over a range of doses.
METHODS
Safety and efficacy data from five double-blinded, placebo-controlled,
multiple-dose studies with MVC alone, and one drug-drug interaction study
with MVC and oral contraceptives (shown not to affect exposure to MVC in
plasma) were pooled.
The studies reported here included a total of 259 healthy volunteers and
HIV-positive patients who received MVC at QD and BID doses ranging from
3 mg BID to 1200 mg QD or placebo (Table1).
Some volunteers received more than one dose of MVC/placebo and are
therefore included in more than one dose group in tables and figures.
Doses are given as unit dose, i.e. actual dose taken at any time point and not
total daily dose.
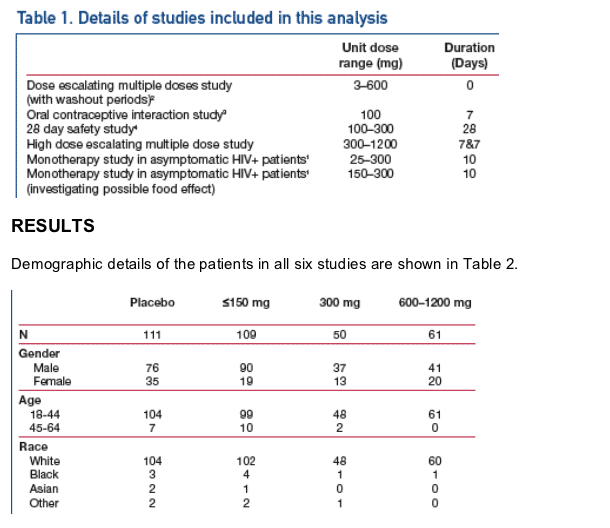
Adverse Events (AEs)
Only headache, nausea and flatulence occurred significantly more frequently
in the MVC 300 mg group (dose being evaluated in Phase 2b/3 trials) vs
placebo.
At high doses of MVC, conjunctival hyperaemia (MVC ≥900 mg) and
blurring of vision (MVC ≥600 mg) occurred. This was not considered
clinically important and did not lead to discontinuation.
The most frequent treatment-related AEs are shown in Table 3.
Postural hypotension was the dose-limiting AE in Phase 1 studies,2 but only
occurred more frequently in patients receiving MVC than patients receiving
placebo at unit doses of ≥600 mg (Figure 1).
Dizziness also only occurred more frequently in patients receiving MVC than
placebo at unit doses of ≥600 mg (data not shown).
Serious AEs and Discontinuations
No serious AEs were reported in any of the six trials.
19 subjects discontinued treatment (Table 4).
11 subjects on MVC:
--Nine in 154 healthy volunteers in four Phase 1 studies (three related)
--Two in 66 HIV+ patients in two Phase 2a studies (not related)
--"no longer willing to participate" - 25 mg QD
--"headache" - 100 mg QD fasted.
Eight subjects on placebo:
--Eight in 95 healthy volunteers in four Phase 1 studies (two related)
--None in 16 HIV+ patients in two Phase 2a studies.

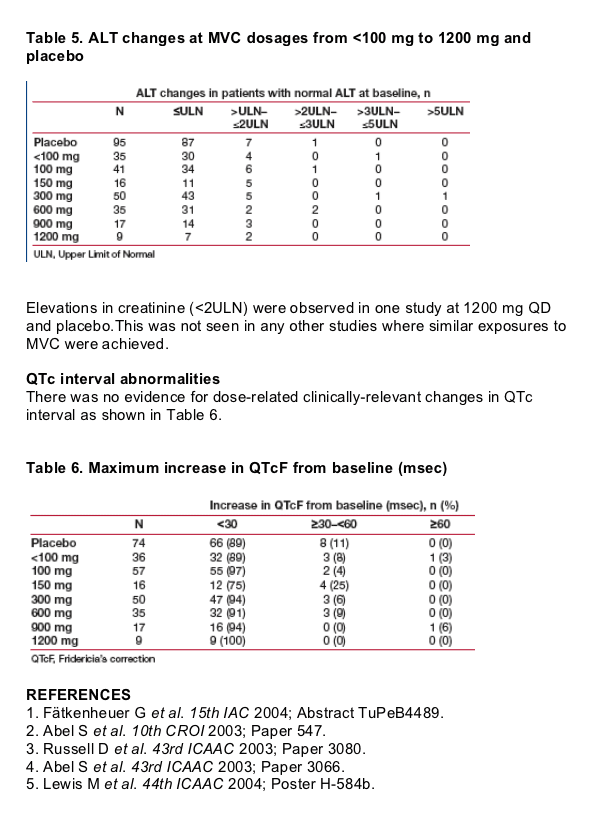
Laboratory abnormalities
Clinically relevant elevations in transaminases occurred sporadically, with no
dose relationship (Table 5) and no associated elevations in bilirubin (data not
shown).
|
|
| |
|
 |
 |
|
|