 |
 |
 |
| |
Entecavir 3-Year Update on Efficacy & Safety
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, Boston, MA
Here is the presentation--
"Entecavir Maintained Virologic Suppression Through 3 Years of
Treatment in Antiviral-Naive HBeAg(+) Patients (ETV 022/901)"
T. Chang1; Y. Chao2; S. Kaymakoglu3; H. Cheinquer4; M. Pessoa5; R. Gish6; F. Poordad7; J. Yang8; H. Brett-Smith7; R. Hindes8
1. National Cheng Kung University Medical College, Tainan, Taiwan.
2. Tri-Service General Hospital, Taipei, Taiwan.
3. Department of Gasterohepatology, Istanbul Medical Facility, Istanbul, Turkey.
4. Universidade Federal Do Rio Grande Do Sul, Porto Alegre, Brazil.
5. Instituto De Infectologia Emilio Ribas, Hospital Dia Av. Dr. Arnaldo, Sao Paulo, Brazil.
6. California Pacific Medical Center, San Francisco, CA, USA.
7. Department of Hepatology and Liver Transplantation, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
8. Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, CT, USA.
Introduction
Entecavir 0.5 mg was superior to lamivudine 100 mg for virologic, histologic and biochemical enpoints at week 48 in nucleoside-naive, HBeAg(+) Chronic Hepatitis B (CHB) patients (ETV-022)1
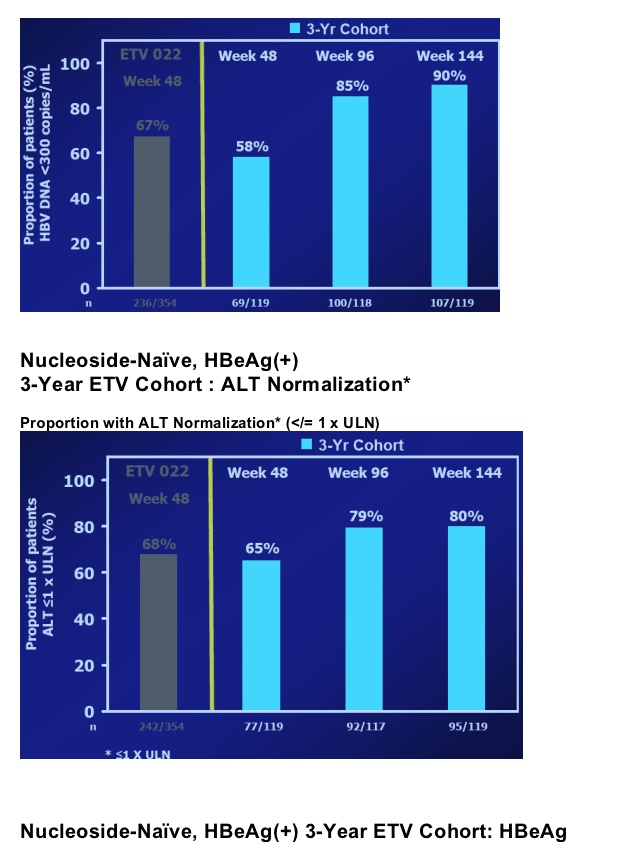
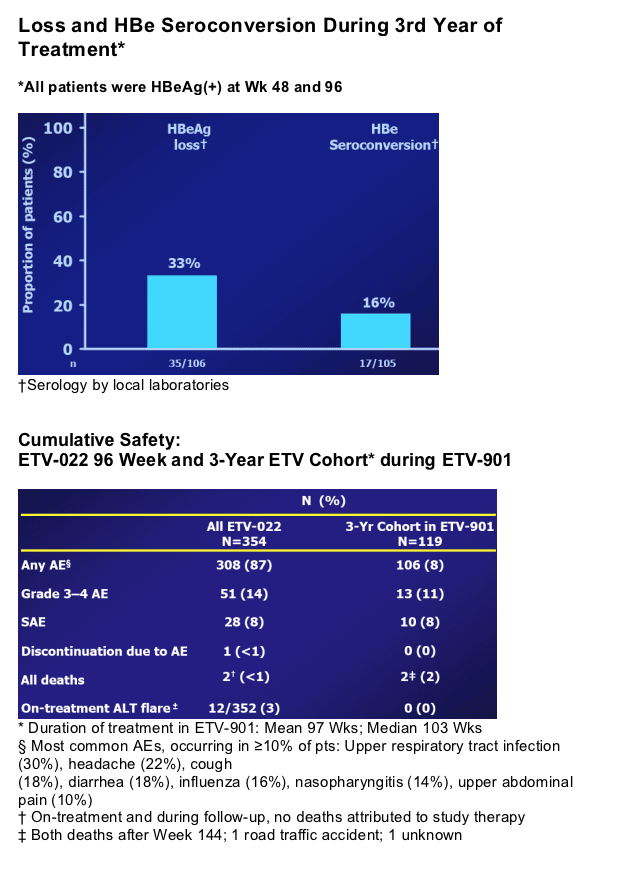
In year 2, patients treated with ETV continued to benefit in terms of VL reduction, ALT normalization and HBe seroconversion2; ETV-022 ended at treatment week 96
Eligible patients based on virologic and serologic status could continue ETV treatment in rollover study ETV-901 (1.0 mg)
We present efficacy and safety results in a cohort from study ETV-901 who received 3 years of ETV
1. Chang TT, et al. N Engl J Med. 2006;354:1001-1010
2. Gish R, et al. Hepatology 2005; 42(4S1): 267A
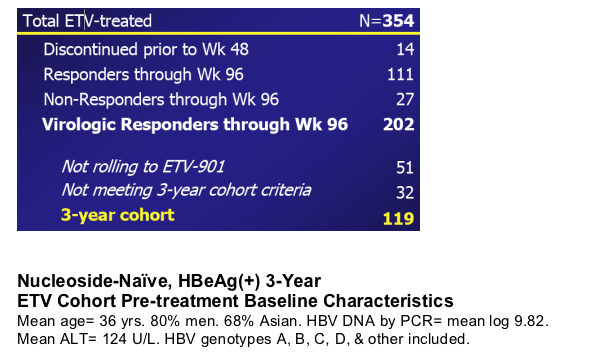
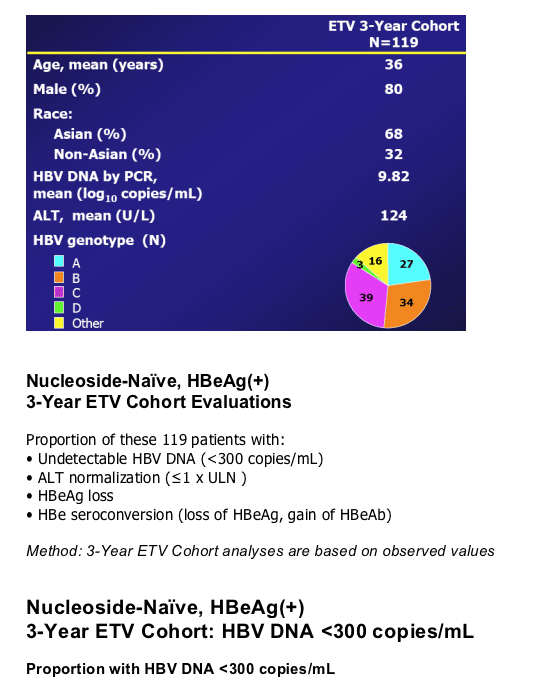
Nucleoside-Naive, HBeAg(+) 3-Year ETV Cohort
The 3-year cohort consists of 119 patients initially treated in ETV-022 who:
- Continued on-treatment in ETV-022 through Year 2, had HBV DNA <0.7 MEq/mLby bDNA (0.7MEq/mL = 700,000 copies/mL by PCR) and remained HBeAg(+) at Week 96 (end of ETV-022)
- Enrolled in ETV-901 with ≦35 day gap in treatment between ETV-022 and ETV-901
- Had HBV DNA measurements by PCR at Week 144
Disposition of ETV-Treated Patients (Studies 022/901)
Total treated with ETV, n=354
Discontinued prior to week 48: 14
Responders through week 96: 111
Non-responders through week 96: 27
Not rolling to ETV-901: 51
Not meeting 3-year cohort criteria: 32
3-year cohort: 119
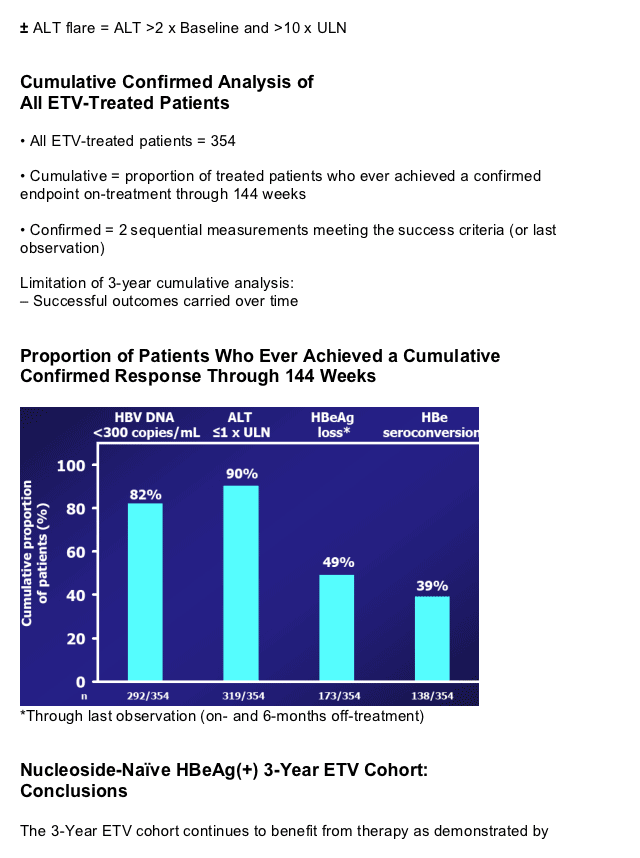

|
| |
|
 |
 |
|
|