 |
 |
 |
| |
Current status of Subjects Receiving Pegays and Ribavirin Follow-On Therapy After 28 Days Treatment with the Hepatitis C Protease Inhibitor Telaprevir (VX-950), Pegasys & RBV
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
Maribel Rodriguez from San Juan, PR was the lead author.
Study VX04-950-102 was designed to provide information on safety of telaprevir when administered over 28 days in combination with PegIFNa-2a and RBV. The clinical and antiviral data during the telaprevir dosing period were presented previously. The authors report here the HCV RNA data during the off-study Peg/RBV treatment period in subjects initially enrolled in Study VX04-950-102.
AUTHOR CONCLUSIONS
12/12 subjects had undetectable HCV RNA at the end of study dosing and continued Pegasys/RBV therapy.
9/11 subjects treated initially with VX-950 in combination with Peg/RBV and who continued Peg/RBV maintained undetectable HCV RNA through week 24 in the off-study Peg/RBV treatment period.
-- 1 subject lost to follow-up
Viral sequencing suggest that:
-- variants (muations) are detected transiently early in treatment but remained undetectable during follow-on treatment
-- in the 2 patients with breakthrough, detection of both WT & R155K suggests that breakthrough may be related to a poor response to Peg/RBV
-- it will be important to evaluate longer durations of telaprevir treatment
The safety & efficacy of a VX-950 (telaprevir) based regimen in longer regimens is currently being evaluated in Phase 2 Studies (PROVE1 & PROVE2).
OBJECTIVES
To evaluate the durability of the virologic response in 12 genotype 1 infected subjects who initially received a 28-day telaprevir-based regimen and who continued PegIFN-a-2a/RBV off-study.
METHODS
Study 102 enrolled 12 treatment-naive subjects with chronic genotype-1 HCV infection. After 28 day study dosing, off-study treatment with Pega-2a/RBV for up to 48 weeks was offered and accepted by all subjects.
Following telaprevir treatment
-- on-study HCV RNA assessments were performed at 7 days, and 12 weeks after the completion of telaprevir-based 28-day treatment.
-- additional HCV RNA assessments were performed at the discretion of the treating physicians during the Peg/RBV therapy. These included assessments at 4, 8, and 24 weeks post-study treatment and later timepoints.
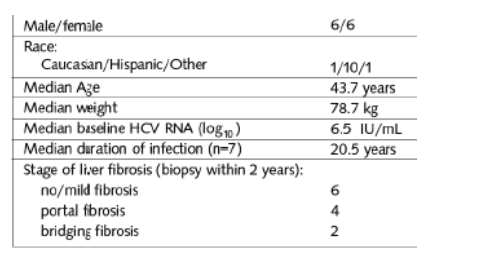
Table 1. demographics & Baseline Characteristics
Baseline HCV RNA was 6.5 IU/mL logs. Six patients had no/mild cirrhosis; 4 had portal cirrhosis; 2 had bridging cirrhosis. Median weight was 78 kg, about 180 lbs.
DISPOSITION of the Subjects
All subjects:
Agreed to start standard therapy with Pegasys/RBV at the end of the 28 days of study dosing.
Completed the 12 week follow-up on-study (ie, after the first dose of telaprevir.
Completed the 24-week followup on Peg/RBV, except 1 lost to follow-up.
As of Sept 30, 2006:
Median time on Peg/RBV was 37 (18-37) weeks.
4 subjects discontinued Peg/RBV-
-- 1 subject was lost to follow-up
-- 2 subjects had detectable HCV RNA
-- 1 subject stopped Peg/RBV at week 18 and has maintained undetectable HCV RNA through week 24
8 subjects continued on Peg/RBV and had undetectable HCV RNA at week 24.
RESULTS
All 12 patients on the triple therapy had <10 IU/mL after 28 days on therapy. They then started Peg/RBV therapy. At the end of the 12-week follow-up period, at week 18, 1 patient had 490 IU/mL and at week 24 had 486 IU/mL. A 2nd patient was lost to follow-up after week 12. A 3rd subject discontinued therapy, but their HCV RNA was negative 6 weeks post treatment. A 4th patient had 121,000 IU/mL at week 24. That left 8 patients who remained undetectable on Peg/RBV at week 36.
At 24 weeks after the start of Peg/RBV:
9 subjects had undetectable HCV RNA
2 subjects had undetectable
Baseline characteristics of subjects in whom variants (mutations) were detected during off-study treatment. These are the two patients described above who had detectable HCV RNA on Peg/RBV follow-on therapy between weeks 12 & 24:
1. Hispanic; female; weight: 101 kg, BMI: 41 kg/m2; viral load I think at baseline: 6,640,000.
2. Hispanic; male; weight: 92 kg, BMI: 31; viral load I think at baseline: 5,910,000
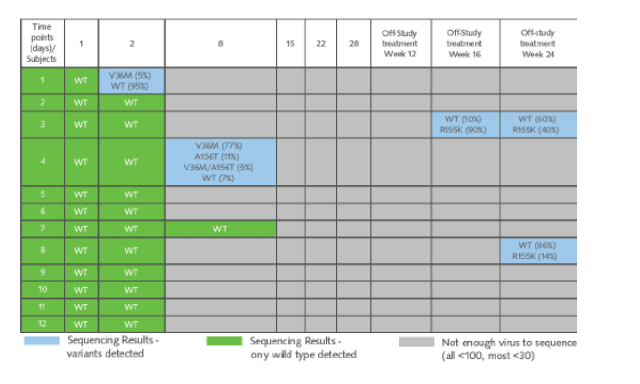
Table 3. Viral Sequencing Results
In the last column you can see the two patients described above who had detectable HCV RNA on Peg/RBV. At week 24 they had 40% R155K and 14% R155K, respectively. Patient 4 had several mutations at week 8 but remained with undetectable HCV RNA at week 36, but the patient did not have 155 mutation (V36M, 77%; A156T, 11%; V36M/A156T, 5%; WT 7%).
|
| |
|
 |
 |
|
|