 |
 |
 |
| |
Adefovir 3 & 5 Years Followup HBeAg+: safety & efficacy
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
"Long-term Efficacy and Safety of Adefovir Dipivoxil for the Treatment of HBeAg-Positive Chronic Hepatitis B (CHB) Patients in Study GS-98-437"
and
"Efficacy and safety of three years therapy with Adefovir (ADV) in Chinese subjects with HBeAg positive chronic hepatitis B (CHB)"
P Marcellin,1 TT Chang,2 SG Lim,3 W Sievert,4 M Tong,5 S Arterburn,6 K Borroto-Esoda,6 and S Chuck6
1Hopital Beaujon, Clichy, France; 2National Cheng Kung University Hospital, Tainan, Taiwan Republic of China;
3National University Hospital, Singapore, Singapore; 4Monash Medical Centre, Victoria, Australia;
5Huntington Medical Center Research Institute, Huntington, CA, USA; 6Gilead Sciences, Inc., Foster City, CA, USA
Treatment of 171 patients with HBeAg+ CHB with adefovir dipivoxil (ADV) 10 mg over 48 weeks resulted in significant histological, virological, serological, and biochemical improvement compared to placebo in the fi rst year of this study.1
The long-term efficacy and safety of ADV in HBeAg+ CHB patients was investigated for up to five years.
METHODS
- Entry criteria were HBsAg+ ≥ 6 months, HBeAg+, serum HBV DNA ≥ 6 log10 copies/mL (Roche Amplicor Monitor PCR, LLQ 1000 copies/mL), and ALT 1.2 - 10 X ULN.
- Patients were randomized to receive ADV 10 mg (n = 172), ADV 30 mg (n = 173), or placebo (n = 170) in year 1. At the beginning of year 2, patients started on ADV 10 mg (n = 224) or placebo (n = 215). However, most patients received multiple doses of both placebo and ADV in year 2.
- Patients with confirmed HBeAg loss or seroconversion discontinued from this study and were followed off treatment; thus, only non-seroconverters remained in this study.
- Patients given ADV 10 mg in year 1 who did not seroconvert in years 1 and 2 could enroll in a long-term, safety and efficacy study (LTSES; n = 65) with assessments every 3 months for 3 years. Patients with confi rmed HBeAg loss or seroconversion discontinued from the LTSES and were followed off treatment.
- A two-state Markov model was used to estimate the percentage of all patients with HBeAg loss and seroconversion over time and to account for seroreversion.
In Year 1, no ADV resistance mutations were detected (0/171; 0%)
Through Year 5, 38/65 patients had a confirmed rebound in HBV DNA or were never fully suppressed
13/65 (20%) LTSES patients developed ADV resistance mutations (A181V, N236T) that were first detected at 3.75 years on study
3 LTSES patients developed A181T; however, this mutation does not confer phenotypic resistance to ADV in vitro2
Safety
- No serious adverse events related to ADV.
- Confirmed increase of ≥ 0.5 mg/dL from baseline in serum creatinine occurred in only 6 patients treated with ADV for up to 5 years; two of these patients discontinued ADV due to increases in serum creatinine in LTSES phase of study.
- The fi rst confi rmed increase of ≥ 0.5 mg/dL in serum creatinine occurred after 3.5 years on study.
AUTHOR CONCLUSIONS
In this study of patients with HBeAg+ CHB:
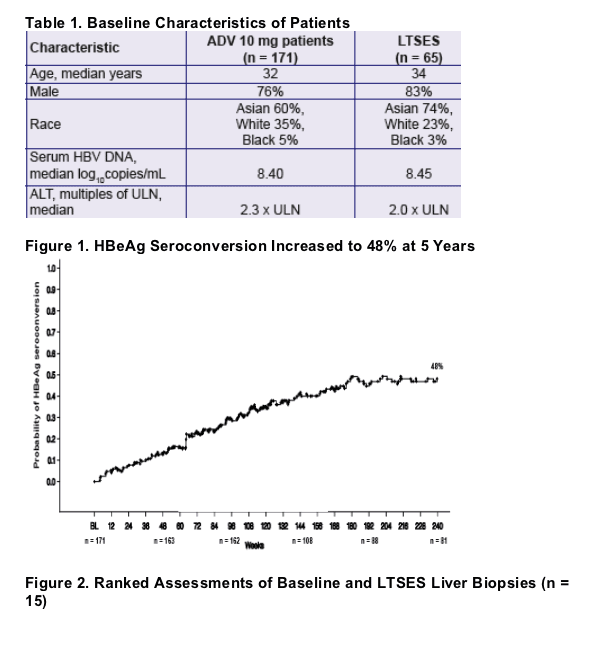
- HBeAg seroconversion increased over time to 48% after 240 weeks on study. Seroconversion was durable in 91% of patients over a median follow up off drug of 3 years.3
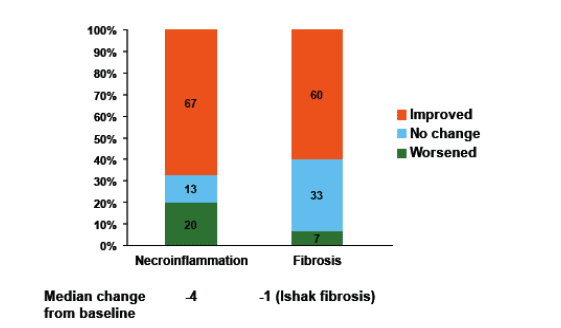
- Liver biopsies at baseline and in the LTSES (n = 15) showed improvements in necroinfl ammation and fibrosis in the majority of patients
- Genotypic resistance occurred in 13/65 (20%) of patients treated with ADV for up to 5 years
- ADV was well tolerated
"Efficacy and safety of three years therapy with Adefovir (ADV) in Chinese subjects with HBeAg positive chronic hepatitis B (CHB)"
YM Mao et al, Renji Hospital, Shangai, PRC
This was a 1 year randomized, double-blind, placebo-controlled study followed by 4 years open label extension study conducted at 12 centers in China. Data are presented for the 3 yrs treatment of the study. In the first 12-week study phase 1 of 3 study group of patients received placebo (2 groups received ADV), and ib the 28-week next phase which was open-label everyone received adefovir. In the third phase, 12-weeks, one of the adefovir groups were switched to placebo. In the final extension all patients received ADV open-label long term.
HBeAg-positive CHB subjects with serum HBV DNA >100,000 c/ml (Roche COBAS AMPLICOR HBV MONITOR Test, LLOD <200 c/ml) and ALT >1xULN were randomized to receive placebo for 12 weeks followed by ADV for 40 weeks or ADV for 52 weeks or ADV for 40 weeks followed by placebo for 12 weeks.
Following 52 weeks, subjects who remain in the study were provided open label ADV 10 mg treatment for a further 208 weeks (4 years). 480 subjects were enrolled in the first year study and 474 and 456 subjects completed the 2nd and 3rd year study respectively. The three treatment groups are designated as PAAA (placebo-ADV-ADV-ADV, AAAA (ADV-ADV-ADV-ADV), and AAPA (ADV-ADV-placebo-ADV).
Efficacy Endpoints
Log reduction in serum HBV DNA, HBV undetectability, ALT normalization, HBeAg loss, HBeAg seroconversion, quality of life as measured by the SF-36 QOL questionnaire.
Samples were analyzed from those subjects with protocol defined HBV DNA breakthrough (HBV DNA level increase >1 log c/ml from the treatment nadir) for the N236T or A181V ADV mutations associated with in vitro ADV resistance.
RESULTS
Median HBV DNA was 8.8 logs in all groups. Median ALT was 2.5 to 2.7 xULN in all groups. Median age was 30 -31 yrs. About 83% were men. 96-99% were HBeAg-positive.
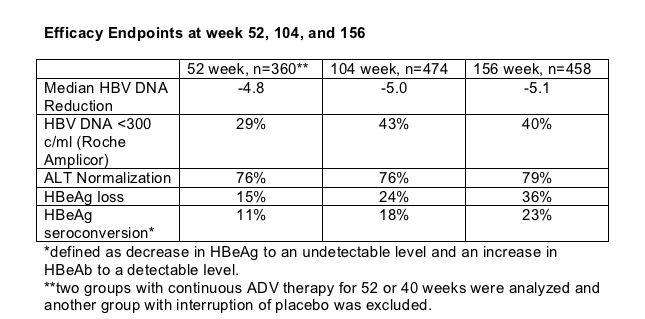
At 3 yrs, 15.4% (74/480) of all the subjects have achieved protocol defined durable HBeAg seroconversion*. Median off drug follow-up was 36 weeks in those 62 patients who stopped ADV therapy once durable HBeAg seroconversiob was achieved. 13 patients restarted treatment due to virologic breakthrough** within 36 weeks. 10% of patients (6/62) seroconverted to HBeAg positive.
*defined as HBeAg loss and gain of HBeAb and HBV DNA <10,000 for any 3 consecutive visits at least 3 months apart from two tests (ie, a response >/=6 months).
**defined as HBV DNA =100,000 c/ml or 0.1 MEq/mL or 0.35 pq/mL by any quantitative assay, or ALT=5xULN with elevated HBV DNA, judged by the investigator as hepatitis B relapse which needed anti-viral treatment and/or return of HBeAg positivity.
Up to 3 yrs, 1% (5/480) subjects (all in AAAA group) demonstrated HBsAg loss. 4 subjects demonstrated HBsAg seroconversion.
At week 52, the groups with continuous ADV therapy showed significant improvement of quality of life. QOL has been further improved at week 104 and sustained at week 156.
SAFETY
Up to 3 yrs, drug-related AEs were reported in 8.2% (39/480) of all si=ubjects, and discontinuation due to AE in only 1.5% (7/480) of all subjects.
Up to 3 yrs, serious AEs (SAEs) were infrequent, occurring in only 5% (24/480) of all subjects. Only 1 of the SAEs were considered by the investigator to be drug-related. There was only 2 fatal SAEs, due to acute hepatitis failure and gastric cancer, respectively.
Up to 3 yrs, 0.,6% (3/480) subjects had a confirmed >0.5 mg/dL increase from baseline in serum creatinine, but only 1 had a peak value out of the normal range.
Resistance
The poster reported mutations were identified in only 3% (14/456) of subjects up to 3 yrs (7 had N236T and 8 had A181V with 1 subject having N236T at year 2 but this was replaced by A181V in the 3rd year), and this was lower than previously described in an HBeAg negative populatiob (8% at 3 yrs).
This study is ongoing and will provide further data on long-term safety and efficacy of ADV in Chinese subjects with HBeAg-positive CHB.
|
| |
|
 |
 |
|
|