 |
 |
 |
| |
Gilead Integrase Inhibitor: Safety and Pharmacokinetics of Single Oral Dose of JTK- 303/GS- 9137, a Novel HIV Integrase Inhibitor, in Healthy Volunteers
|
| |
| |
Isao Kawaguchi*1, Tomohiro Ishikawa1, Motoki Ishibashi2, Shin Irie2, and Atsuyuki Kakee 1
1Japan Tobacco Inc., Tokyo, Japan; 2Kyushu Clinical Pharmacology Research Clinic, Fukuoka, Japan
Author conclusions:
- JTK- 303/GS- 9137 was well tolerated following oral, single dose administration in the fasted state up to 800 mg, and in the fed state at 400 mg.
- Maximum tolerated dose was not detected.
- Cmax and AUC0- inf increased with dose escalation from 100 mg to 800 mg, but the parameters increased in a less than dose- proportional manner.
- The absorption of JTK- 303 significantly (approximately 3- fold) increased in the presence of food.
- Plasma JTK- 303 concentrations at 12 to 24 hours exceeded in vitro protein binding- adjusted EC50.
- These findings in this study support further investigation of JTK- 303 in future clinical studies.
ABSTRACT
Background: JTK- 303/GS- 9137 is a novel HIV integrase inhibitor with potent in vitro anti- HIV activity and is being developed for treatment of HIV infection. A single ascending dose phase I study in healthy volunteers was conducted to assess the safety, tolerability and pharmacokinetics of JTK- 303.
Methods: A single blind, randomized, placebo- controlled single oral dose escalation study was conducted in 32 Japanese male healthy volunteers. Eight subjects (6 active and 2 placebo) per cohort received dose of 100, 200, 400 or 800mg in the fasted state. In addition, the subjects in 400mg cohort also received additional same dose with breakfast after a washout period. For the evaluation of pharmacokinetics, blood samples for determination of JTK- 303 plasma concentrations were collected over 24 hours post- dose. Laboratory safety tests, vital signs and ECG were performed throughout the study.
Results: JTK- 303 was safe and well tolerated with no serious adverse events and no grade 3 or 4 adverse events in any cohort. All adverse events were mild. One subject experienced mild anorexia and another subject experienced mild laboratory abnormalities. No clinically significant ECG changes were noted. In the ascending dose portion, plasma concentrations of JTK- 303 attained Cmax in general at 0.5 to 4 hour post- dose. Cmax and AUC of JTK- 303 increased with dose escalation from 100 to 800mg. Food significantly increased the Cmax and AUC of JTK- 303, approximately 3- fold relative to administration in the fasted state. Plasma concentrations of JTK- 303 at 12 to 24 hours exceeded protein binding- adjusted in vitro EC50 concentrations.
Conclusions: JTK- 303 is orally bioavailable, safe and well- tolerated following single doses. These data support further investigations of JTK- 303, including in HIV- infected individuals.
BACKGROUND
- JTK- 303/GS- 9137 is a novel low molecular weight HIV- 1 integrase inhibitor.
- JTK- 303 has demonstrated potent in vitro anti- HIV- 1 activity against HIV- 1IIIB replication in human peripheral blood mononuclear cells (PBMC). Animal studies showed that JTK- 303 also has a favorable pharmacokinetic profile (Matsuzaki, CROI 2006, Abstract F- 117).
- The objective of this study was to evaluate the safety and pharmacokinetics of JTK- 303 in healthy adult male volunteers in a placebo- controlled single blind study. The effect of food was also to be determined.
METHODS
- This study was a randomized, single blind, placebo controlled, single ascending dose trial in Japanese healthy adult male volunteers.
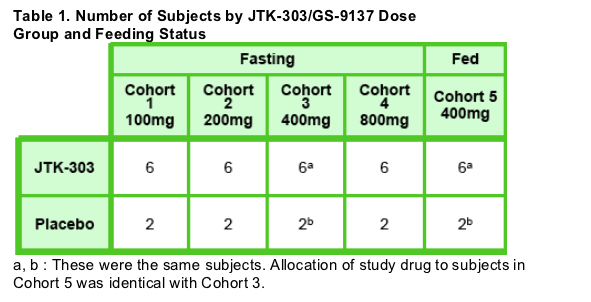
- Thirty- two subjects were to be randomized to one of four dose cohorts (comprising Cohorts 1 to 4) as shown in Table 1. For each step, six subjects were to receive a single dose of JTK- 303 and two were to receive a single dose of matching placebo.
- The subjects who were randomized to Cohort 3 (400mg fast) and received study drug under fasting conditions also received the same study drug under fed conditions in Cohort 5 (400mg fed) after a washout period (> 10 days).
- Adverse events were evaluated throughout the study. Clinical laboratory tests (hematology, blood chemistry and urinalysis), vital signs and 12- lead standard electrocardiograms were also performed.
- Plasma JTK- 303 concentrations were analyzed for the evaluation of pharmacokinetics.
RESULTS
Safety
No deaths, other serious adverse events or other significant medical events were reported in any cohort. There were no subjects discontinued.
- No placebo subject experienced an adverse event (AE) and two subjects who received JTK- 303 reported three AEs. All subjects recovered with no sequelae.
- One subject (800mg fast) : mild anorexia
- One subject (400mg fed) : increased AST and increased ALT(<1.3 x ULN)
Pharmacokinetics
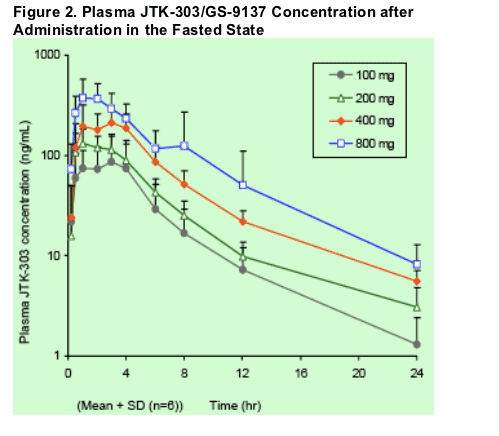
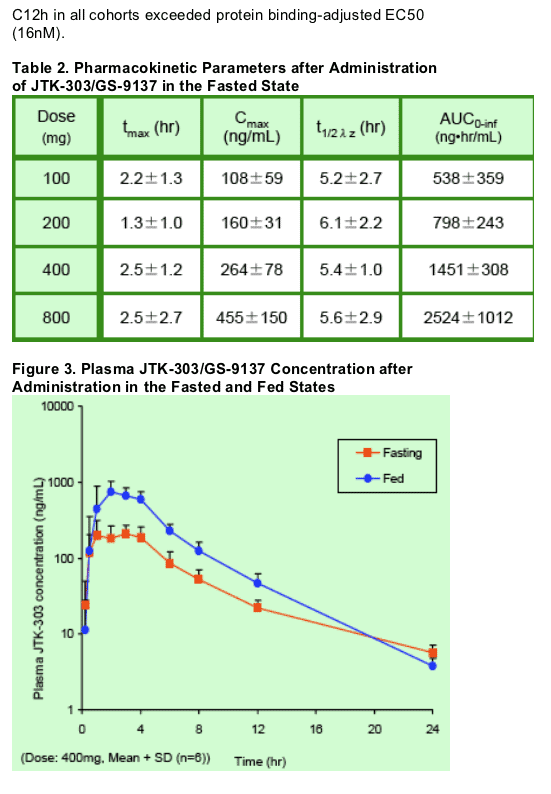
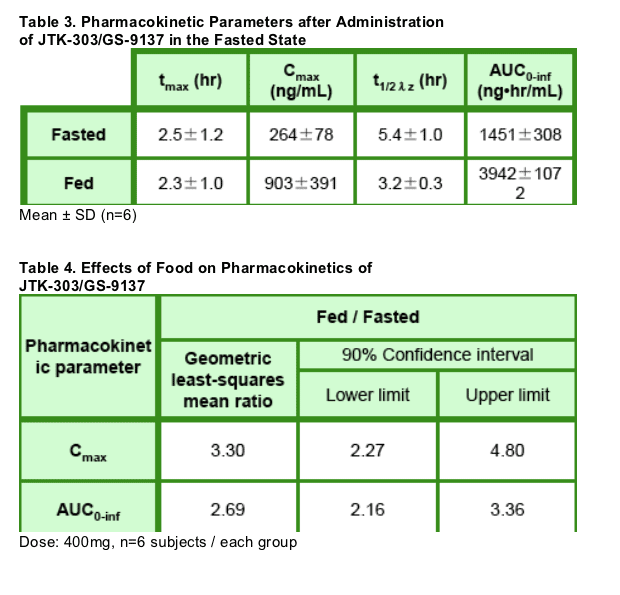
Plasma concentrations and pharmacokinetic parameters of JTK- 303 in the fasted state are shown in Figure 2 and Table 3, respectively.
- Cmax and AUC0- inf of JTK- 303 increased with dose escalation from 100 to 800 mg, but the proportions of these pharmacokinetic parameters were lower than the proportion of dose.
- The absorption of JTK- 303 significantly (approximately 3- fold) increased in the presence of food (Figure 4, Tables 3 and 4 ).
- Mean plasma JTK- 303 concentrations at 12 to 24 hour exceeded in vitro protein binding- adjusted EC50 value (16 nM).
|
|
| |
| |
|
 |
 |
|
|