 |
 |
 |
| |
Prevention of Rectal SHIV Transmission in Macaques by Tenofovir/FTC Combination
|
| |
| |
Reported by Jules Levin
These study results were presented at the 13th CROi in an oral session by Walid Heneine (Laboratory Branch Division of HIV/AIDS Prevention). Another study presented at CROI by Angela Kashuba found tenofovir significantly reduced HIV RNA in the genital tract of men and women, my next report.
"Prevention of Rectal SHIV Transmission in Macaques by Tenofovir/FTC Combination"
Author Summary and Conclusions
Tenofovir/FTC combination protected all 6 treated animals from infection after 14 repeated rectal SHIV exposures.
Treated animals remained uninfected despite receiving seven times more exposures than controls (median exposures to infect controls is 2).
Data suggest that chemoprophylaxis with a potent antiretroviral drug combination can be highly effective in preventing sexual HIV transmission.
The observed protection by tenofovir and FTC may not reflect that of Truvada, because a higher dose of tenofovir was used.
Ongoing study with FTC alone shows that 4 of 6 animals are protected after 10 exposures suggesting that FTC contributes substantially to the protection level of Tenofovir/FTC combination.
In the Cox proportional hazards model control monkeys are 5.7 times more likely to become infected than treated monkeys (p-value is 0.024)
Conclusive data when study is completed.
Adding tenofovir to FTC at a dosing similar to that in Truvada may enhance further the protection.
Drug potency matters for preventing sexual infection by chemoprophylaxis
INTRODUCTION
In the absence of an effective vaccine, chemoprophylaxis with antiretrovirals has considerable potential for preventing HIV-1 transmission.
Macaque models show that tenofovir can provide substantial protection against parenteral or mucosal virus exposures.
However, this protection may be reduced at lower drug doses equivalent to those used in humans.
Current human trials with tenofovir will ultimately determine the efficacy of this intervention.
Many potent antiretrovirals with favorable safety and pharmacodynamic profiles are now available and are good candidates for chemoprophylaxis.
Can these drugs fully protect against sexual transmission?
What is the relationship between drug potency and protection?
Higher drug potency provides more protection?
We determined whether the increased potency in a combination of 2 RT inhibitors, tenofovir and FTC, protects macaques from repeated virus exposures.
Materials and Methods
Tenofovir (PMPA, injectable), active form of oral tenofovir disoproxil fumarate, TDF, Viread.
FTC (Emtricitabine, brand Emtriva; injected FTC),
(Oral Truvada = TDF and FTC)
Six male Rhesus macaques injected subcutaneously with tenofovir 22 mg/kg and FTC 20 mg/kg once daily- initiated 9 days before virus challenge. FTC dose similar to that used in humans, TDF dose a little higher than that used in humans.
Six male control macaques received no drug treatment.
Repeated Exposure Model Used
Model uses more physiologic virus inoculum- 10 TCID (3.8 105 virus particles equivalent); virus particle level similar to that seen in acute infection in seminal fluid. SHIV162p3 contains an HIV-1 R5 env.
Repeated weekly rectal virus exposures- 14 total exposures.
Infection was monitored by serology, RT-PCR of plasma and proviral PCR of PBMC.
Animals considered protected if seronegative and PCR negative.
Data from both 6 realtime and 9 historical controls were used.
RESULTS
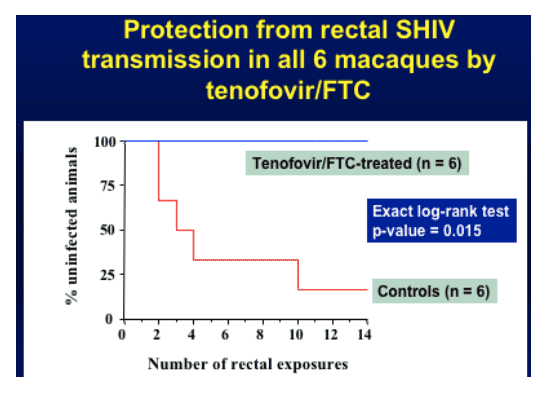
Red line in graph below (control animals, did not get treatment but were exposed to HIV) gets HIV-infected on average after 4 exposures (rectal viral exposures), some earlier. Previous data suggests a median of 2 exposures results in HIV-infection. Treated (Tenofovir/FTC) animals (blue line) showed no evidence of HIV-infection after 14 exposures. Drug treatment continued for 4 weeks after last exposure & animals remain antibody negative & PCR negative.
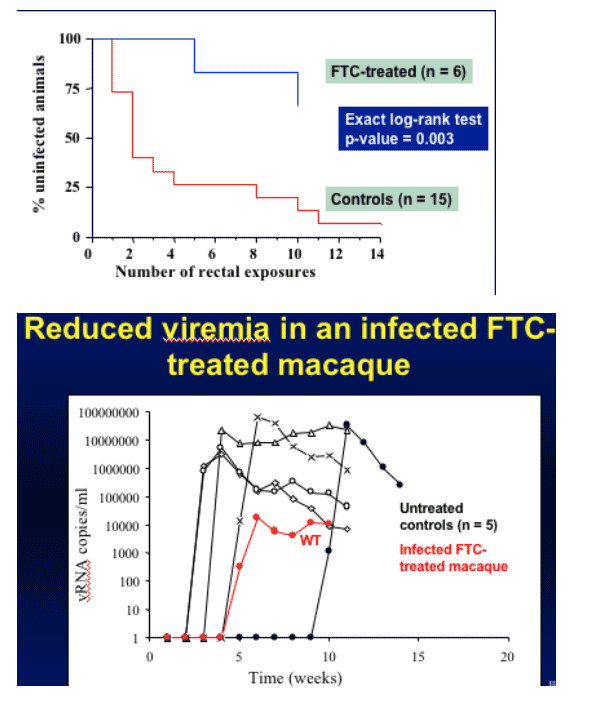
FTC alone was studied with up to 10 HIV exposures, since FTC has certain benefits. Only one animal got HIV-infected at week 5 and a second one was infected at week 10. There was a big and significant difference between group receiving FTC and animal control group who did not receive FTC. They have viral load data on the first animal, who was infected with HIV after receiving FTC. HIV viral load was reduced by at least 3-log during 5 week observation period so far. Viruses remain wild-type and up to 5 weeks so far virus remains wild-type with no M184V.
Protection from rectal SHIV transmission in all 6 macaques by tenofovir/FTC (no HIV-infection resulted)
Chemoprophylaxis with FTC Alone
How much does FTC (Emtricitabine, Emtriva) contributes to the observed protection?
FTC is very potent (-1.8 decrease in virus load)
FTC is well tolerated, good safety profile, once daily dosing, long intracellular half-life (40 h).
Pharmacokinetic analysis suggests that 20 mg/kg/day in rhesus macaques is equivalent to the dose used in humans.
Evaluated protection by FTC in six Rhesus macaques.
High level protection from rectal SHIV transmission by FTC alone (HIV-infection resulted in 2/6 macaques but HIV viral load was significantly reduced)
|
|
| |
| |
|
 |
 |
|
|