 |
 |
 |
| |
"Week 12 Antiviral Activity of Higher Doses of albumin interferon alfa-2b (Albuferon) Combined with Ribavirin in Non-responders to Prior Interferon Based Therapy for Chronic HCV-Infection"
|
| |
| |
Reported by Jules Levin
DDW May21, 2006, Los Angeles
Authors: David Nelson (University of Florida, Gainesville) et al. Study sponsored by Human Genome Sciences.
Author Summary of Efficacy
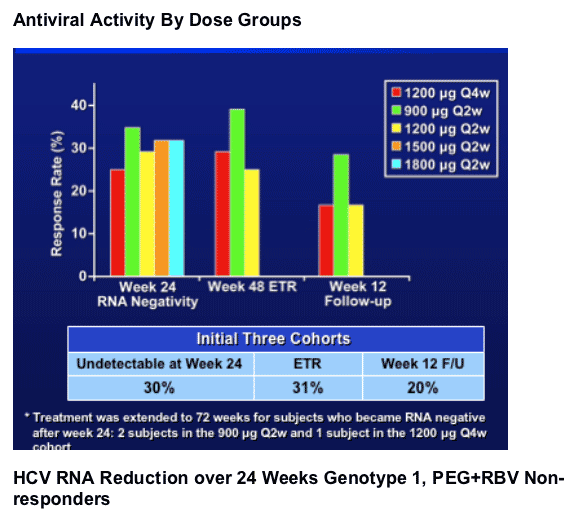
- week 48 RNA negativity rate (ETR) was 31% in the 900-1200 ug dose cohorts.
- The preliminary SVR rate is 20% based on wk12 FU. The relapse rate to date is low.
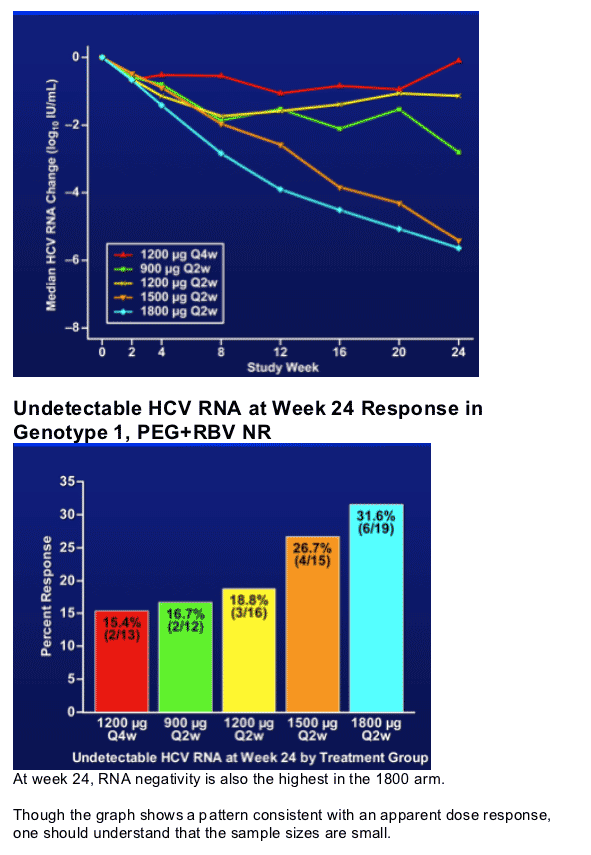
- The 1800 ug cohort shows the greatest w24 HCV RNA negativity rates in genotype 1, PEG+RBV non responders.
- Few viral breakthroughs were observed. The 1200 ug Q4w is able to maintain RNA negativity.
Author Summary of Safety
48 week safety profile is acceptable for the 900/1200 ug cohorts:
- no increase in AE between weeks 12-24-48
- hematologic reductions stabilize by week 8
- immunogenicity rates are low and there are no apparent clinical corrections
- Q4w appears to be better tolerated (less hematologic reductions)
24+ week safety profile is acceptable for the 1500 ug cohort.
12-24 week safety profile for the 1800 ug cohort is comparable to the lower dose cohorts.
In summary, no dose response observed - and the 1800 mcg Q2w was surprisingly well tolerated in this IFN experienced non-responder population.
Caveat: this population is self-selected for highly motivated patients who previously tolerated IFN.
Author Conclusions
Antiviral activity is promising in a non-responder population:
- ETR rate is 31% (22/71) in the 900-1200 ug cohorts
- Viral response at w12 followup after ETR is 14/71 (20%)
- The 1800 ug arm shows maximal activity at week 24 in genotype 1 PEG+RBV non-responders
Long-term safety profile is favorable:
- no significant increase in severity of adverse events between week 12 and 24
- hemoatologic reductions stabilized by week 8 and are well managed with dose reductions
- little drug accumulation between week 12 and 24
- no significant increase in severity or number of adverse events with increased dose of alb-IFN
We look forward to presenting SVR data and more long term safety from the higher dose cohorts over the coming year.
The higher dose arms, esp 1800 mcg is indeed interesting given the available antiviral response data in a very refractory patient population.
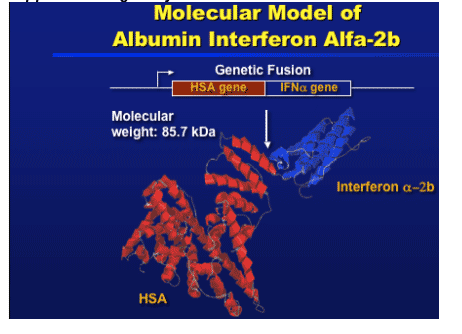
Albumin interferon alfa-b (alb-IFN) is a single polypeptide molecule that combines the therapeutic activity of interferon alpha with the long half-life of human serum albumin. Albuferon is a novel 85.7 kilodalton recombinant protein consisting of interferon alpha genetically fused to human serum albumin. Recombinant human albumin is a carrier protein with no intrinsic activity, but with a long circulating half life, and human serum albumin is expected to extend systemic circulation of recombinant interferon alpha 2b and improve its therapeutic activity. The long half life of the drug (~150 hours or 6 days) supports dosing every 2 or 4 weeks.
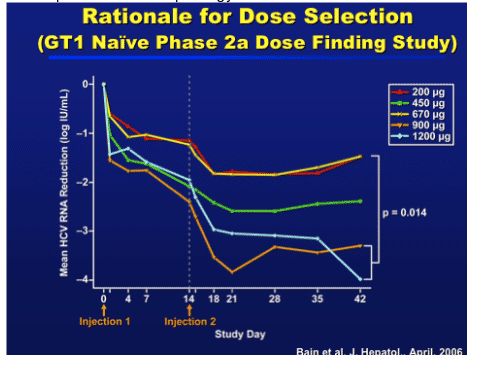
Dose selection for combination Phase 2 studies were based on the Phase 2a study were doses of 900 and 1200 mcg administered 14 days apart showed:
1. Mean antiviral reduction of 3.2 log IU/mL at day 28 with 69% having a > 2 log reduction
2. Antiviral response was maintained 28 days after the 2nd dose, indicating that a Q4w regimen may be feasible.
3. Also published in J Hepatology 2006
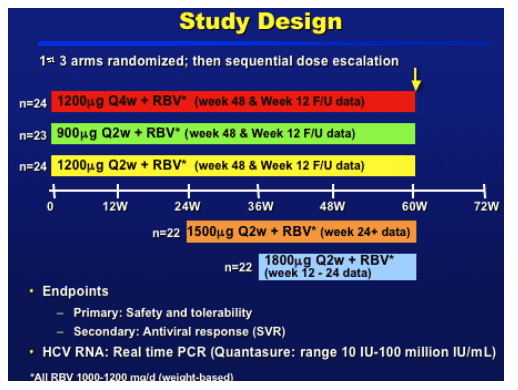
STUDY DESIGN
Dose escalation after the MB reviewed 8 weeks of safety data from all subjects enrolled in the previous cohort.--
1500 mcg - after safety from the 900-1200
1800 mcg - after safety from the 1500 mcg and cummulative safety from the 900-1200
As more lab data is available compared to AE data, we are presenting 24w of lab data for the 1500-1800 arms and 60w for the 900-1200. AE data till week 12 for the 1800 and till week 24 for the 1500.
Phase 2 Non Responder Study
Definition of Non-Responder to IFN Therapy:
- lack of ETR (failed to clear HCV RNA on therapy)
- at least 12 weeks of therapy with lack of EVR
- at least 50% should be PEG-IFN+RBV NR
- no limitation on the number of prior therapies
Baseline Characteristics
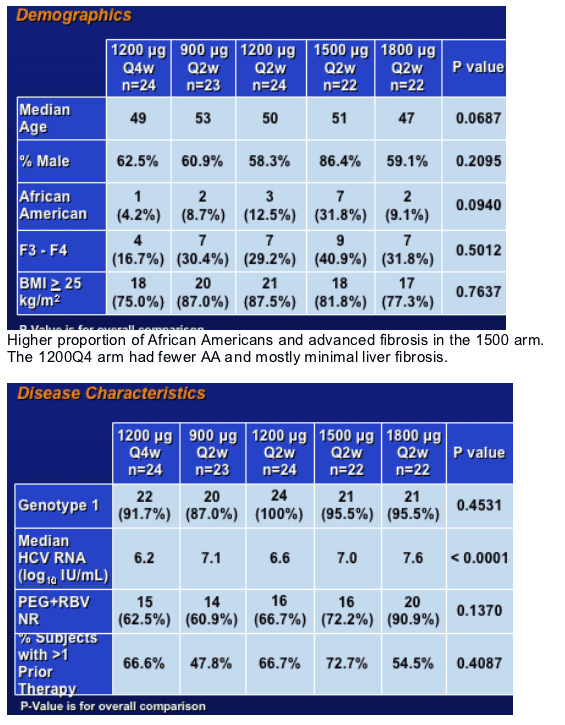
Higher baseline HCV RNa esp in the 1800 mcg cohort. Also, the greatest number of PEG+RBV non responders that were mostly genotype 1.
Also, the majority of patients had been exposed to more than one prior IFN containing regimen.
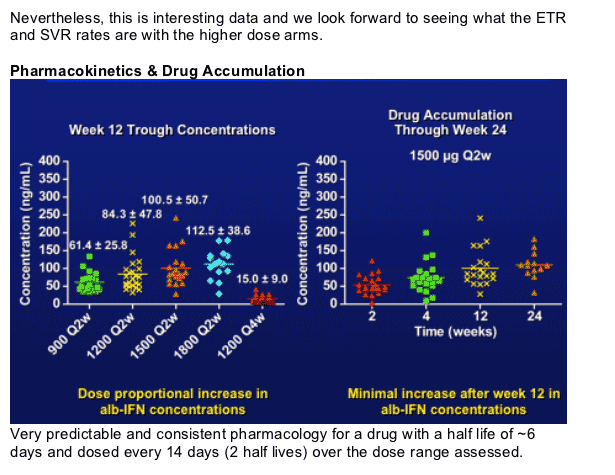
Dose proportional increases in trough concentrations with the Q2w arms.
Accumulation of 70-90% and steady state levels would be predicted after the 3rd dose. Clearly minimal accumulation after week 12 (based on the sampling at 0, wk 4-12-24-48).
Safety & Tolerability
Well tolerated with the most common adverse events of fatigue (50%), headache (34%), arthralgia (24%) and myalgia (21%):
- no significant increase in severity over the first 24 weeks
- the incidence was similar across the 5 cohorts
10.4% (12/115) required dose reductions for AEs and 5.2% (6/115) required discontinuations for AEs.
4 serious adverse events: brain aneurysm, abdominal pain (LUQ), ethylene glycol toxicity, appendicitis.
As this is primarily a safety study, the safety aspects were looked at critically.
Overall, the tolerability was favorable and there were no surprises in terms of the type of AE's.
Duration of AE's as well as severity - no increase with dose for all the Q2w arms. Q4w is probably the best tolerated.
Ethylene glycol ingestion was likely due to social stressors - DUI and impending divorce and jail time. No prior history of depression with normal CESD scores.
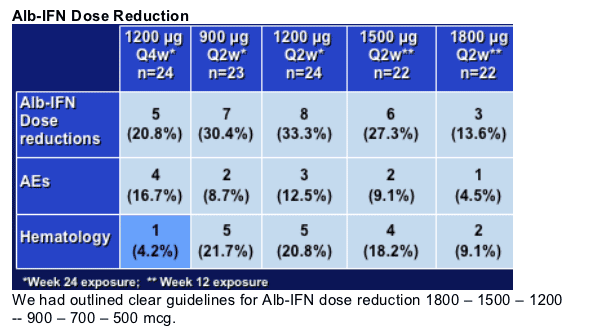
In particular, dose reduction was initial response for ANC<750, platelet<50,000; GCSF use allowed if ANC<500
The dose reduction strategy worked well for all dose groups - again no dose response, and for the few patients who required GCSF, there was a robust bone marrow response limiting the need for GCSF to a few doses.
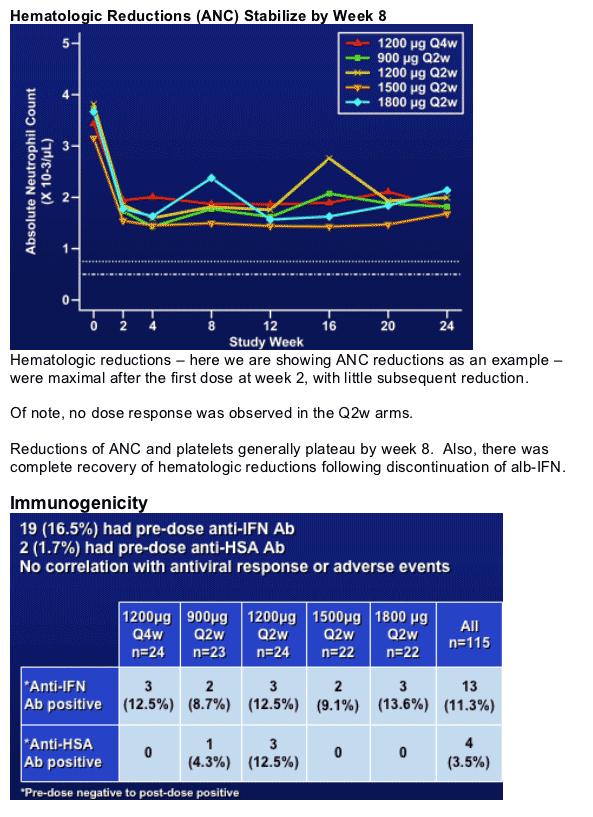
The frequency of patients who had pre-dose Ab to IFN was 16.5% and was similar to that observed in the Phase 1-2 study conducted in an IFN experienced population.
Emergent antibody titers to IFN or HSA were low and did not increase over time (ie between week 12-48). Very few patients (2) had neutralizing antibodies.
No correlation with antiviral response, AE's, PK or with lab parameters
|
| |
|
 |
 |
|
|