 |
 |
 |
| |
A COMPARATIVE TRIAL OF TELBIVUDINE AND ADEFOVIR DIPIVOXIL FOR TREATMENT OF HBeAg-POSITIVE, COMPENSATED CHRONIC HEPATITIS B: 24 WEEK RESULTS
|
| |
| |
Reported by Jules Levin
DDW, May 21-25, Los Angeles
Authors: E J Heathcote, HL-Y Chan, C-L Lai, M Cho,
Y-M Moon, Y-C Chao, R Myers, G Minuk,
P Marcellin, L Jeffers, W Sievert, R Kaiser, G Chao,
G Harb, N Brown, and the 018 Study Group
Author Summary: Telbivudine vs Adefovir at 24 Weeks
Significantly greater viral suppression with telbivudine;
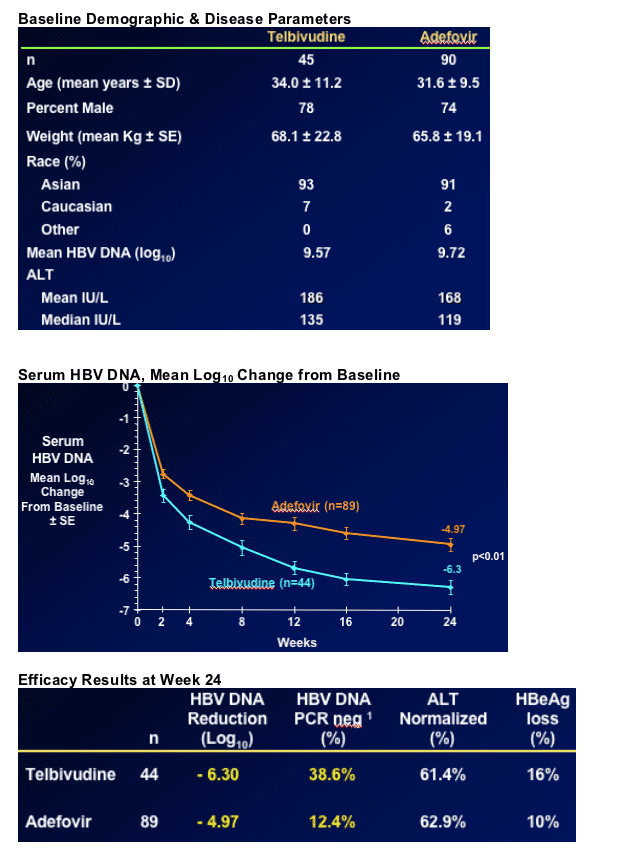
B Quantitative reduction of HBV DNA (-6.3 vs -4.97 logs)
B HBV DNA non-detectable by PCR (38% vs 12% <300 copies/ml)
More rapid viral suppression with telbivudine - difference vs. adefovir evident after two weeks (see graph below)
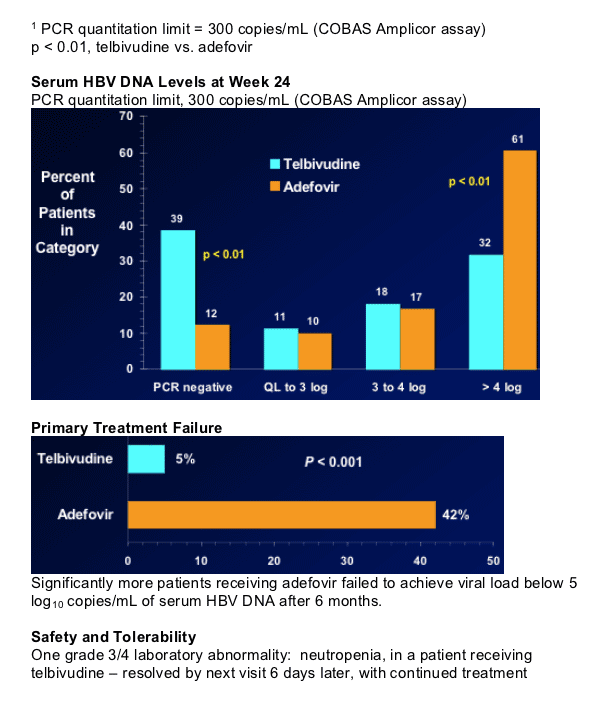
Significantly more adefovir-treated patients with viral load
> 5 log after 6 months
Both treatments well tolerated.
Background
- Profound viral suppression: important objective for treatment of chronic hepatitis B
- For oral antivirals, efficacy and resistance depend on rapid early suppression of HBV DNA
- Comparative trials of antiviral therapies needed to establish relative therapeutic efficacy and safety
Telbivudine: Clinical Profile To Date
Phase II and III
Significantly greater efficacy outcomes compared with lamivudine: 1,2
- HBV DNA suppression and clearance to PCR non-detectable
- Protocol-defined "Therapeutic Response"
- HBV DNA < 5 log10 and: HBeAg loss OR ALT normal
- Histologic improvement
- Resistance
1 Lai et al., Gastroenterology 2005; 129:528-536; 2 Lai et al., AASLD 2005
Today:
RCT - Telbivudine vs. adefovir dipivoxil, HBeAg-positive CHB
Study Design
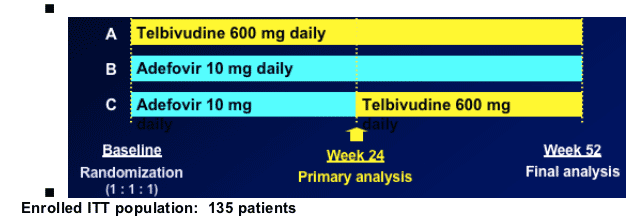
018 Trial: Telbivudine vs. Adefovir in HBeAg+ Chronic Hepatitis B Patients
Randomized, open label, multicenter trial
Key entry criteria:
-- HBeAg positive, treatment-na´ve, compensated CHB
-- HBV DNA >106 by COBAS Amplicor PCR (LLD 300 cp/mL); ALT 1.3-10 xULN
Efficacy Endpoints
018 Trial: Telbivudine vs. Adefovir in HBeAg+ Chronic Hepatitis B Patients
Primary efficacy endpoint:
B Serum HBV DNA reduction at Week 24
Secondary efficacy endpoints:
B Clearance of HBV DNA to PCR non-detectable
B ALT normalization
B _ HBeAg loss
Treatment failure (and resistance):
B Primary treatment failure: serum HBV DNA never below 5 log
B _ Resistance - to be assessed at 1 year
|
| |
|
 |
 |
|
|