 |
 |
 |
| |
Growth Factors Versus Dose Reduction for Pegylated Interferon alfa-2b and Ribavirin-Associated Neutropenia and Anemia in HIV/HCV Co-Infected Patients
|
| |
| |
Reported by Jules Levin
DDW, May 2006, Los Angeles
J. S. Kadam,1,2 K. Jones,3 R. Peterson,1,2 L. Dove,4 D. Pearce,5 T. Hassanein,6 L. Doonquah,7 M. Glesby,8 L. Heller,9 D. Aboulafia,10 J. Rodriguez,11 H. Bonilla,12 J. Galpin,13 J. Aberg,14 B. Johnston,15 R. Liu,4 I. M. Jacobson,1,2 A.H. Talal1,2
1Division of Gastroenterology and Hepatology, Department of Medicine, and 2Center for the Study of Hepatitis C, and 3Department of Psychiatry, Weill Medical College of Cornell University, New York, New York; 4Columbia University, New York, New York; 5AltaMed Health Services Corp., Los Angeles, California;
6SCTI Liver Center Research Foundation, Riverside, California; 7Family Medical Counseling Services Corp., Washington, DC; 8Division of Infectious Diseases, Weill Medical College of Cornell University, New York, New York; 9Schering Plough Corp., Kenilworth, New Jersey; 10Virginia Mason Medical Center, Seattle, Washington;
11Orange Coast Medical Group, Newport Beach, California; 12Summa Health Systems, Akron, Ohio; 13Shared Medical Research Foundation, Tarzana, California; 14Department of Medicine, New York University Medical Center, New York, New York; 15Department of Medicine, Saint Vincent's Medical Center, New York, New York
RESULTS (1)
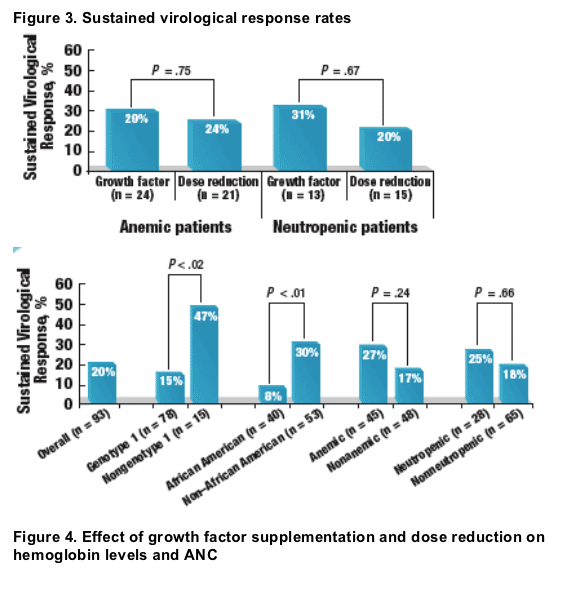
- Overall, 20% of the patients achieved an SVR (Figure 3)
- SVR rates were higher in patients with hematologic abnormalities than in those without, although differences did not achieve statistical significance (Figure 3)
- Growth factor supplementation and dose-reduction strategies resulted in similar SVR rates
- SVR rates were significantly lower in patients with HCV genotype 1 than in those with other HCV genotypes (15% vs 47%, P = .016)
- Among African American patients, only 8% achieved an SVR compared with 30% of non-African American patients (P = .009)
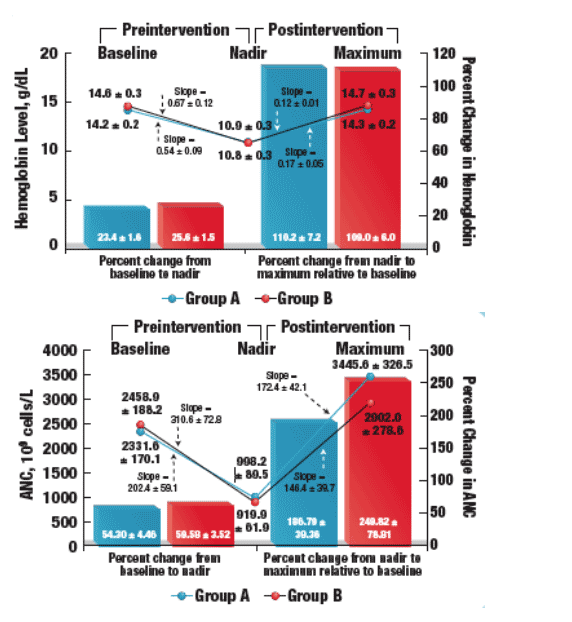
- Recovery of ANC is substantially more rapid than that of hemoglobin levels as indicated by the slope of the maximum recovery line (Figure 4)
- Dose reduction and growth factor supplementation appear to have similar rates of correcting the underlying hematologic abnormality as indicated by the similar slopes
The authors conclude: "Growth factor supplementation and dose reduction may be equally effective for the management of neutropenia and anemia in HIV/HCV-coinfected individuals treated with PEG-IFN alfa-2b and RBV. I say, Jules Levin, the numbers of patients in the final data showing SVR rates were too small to show a significant difference: 29%, n=24 (recd growth factor) vs 24%, n=21 (didn't receive growth factor) (p=.75). Neutropenia: 31% n=13 (receiving growth factor vs 20% n=15 (not receiving growth factor) p= .67).
Author's Conclusions
- This is the first prospective, randomized trial comparing dose reduction with growth factor supplementation using a wide spectrum of weight-based PEG-IFN alfa-2b and weight-based RBV
- Growth factor supplementation and dose reduction may be equally effective for the management of neutropenia and anemia in HIV/HCV-coinfected individuals treated with PEG-IFN alfa-2b and RBV
- The speed of hemoglobin or ANC recovery likely reflects the underlying etiologic mechanism of anemia or neutropenia
- Adding G-CSF or reducing exposure to PEG-IFN likely stimulates direct ANC production; hence, the rapid ANC recovery after either intervention
- Anemia, likely caused by erythrocyte hemolysis induced by intracellular RBV accumulation, probably takes longer to correct because of the indirect effects of erythropoietin or RBV reduction
- SVR percentages were increased in patients with hematologic abnormalities compared with those without these abnormalities
- Larger randomized trials are required to fully evaluate the association between treatment outcome and dose reduction
Background
- Approximately 250,000 people are coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) in the United States
- Pegylated interferon alfa (PEG-IFN) plus ribavirin (RBV), which is the standard therapy for HCV, results in sustained virological response (SVR) rates of 27% to 40% in HIV/HCV-coinfected patients overall and in 14% to 29% of coinfected patients with difficult-to-treat HCV genotype 11-3
- Hematologic abnormalities, specifically anemia and neutropenia, can result in dose reduction in up to 27% of neutropenic patients and 16% of anemic patients2
- Conventionally, PEG-IFN-associated and RBV-associated hematologic abnormalities have been managed by reducing the drug dose, a strategy that may adversely affect SVR rates4
- Recent studies suggest that growth factor support for anemia and neutropenia is effective in maintaining hematologic parameters during HCV treatment
- The efficacy of growth factor supplementation compared with that of dose reduction has not been assessed in coinfected patients
Aim
- To compare growth factor support with a dose-reduction strategy in HIV/HCV-coinfected patients who experience hematologic abnormalities while receiving PEG-IFN alfa-2b (PegIntron®) plus RBV
Methods
Patients
- HIV/HCV-coinfected patients were eligible if they were between the ages of 18 and 70 years
- Exclusion criteria included baseline neutropenia (absolute neutrophil count [ANC] <1.2 x 109 cells/L), thrombocytopenia (platelet count <70 x 103 cells/⊥), anemia (hemoglobin <11.0 g/dL), renal insufficiency (serum creatinine ≥1.7 mg/dL), hepatitis B surface antigen positivity, decompensated cirrhosis or forms of liver disease other than HCV, severe depression or psychosis, uncontrolled seizures, illicit drug use, poorly controlled cardiovascular disease, pregnancy, lactation, diabetes mellitus, or autoimmune disorders
Study Design
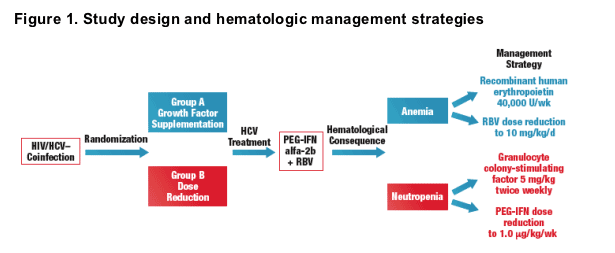
- An investigator-initiated, prospective, open-label, randomized study was conducted between February 2002 and March 2004 at 14 sites throughout the United States to compare the efficacy of 2 strategies-dose reduction versus growth factor supplementation-for managing hematologic abnormalities in HIV/HCV-coinfected patients (Figure 1)
- All patients received PEG-IFN alfa-2b 1.5 _g/kg/wk plus RBV 13 ± 2 mg/kg/day for up to 48 weeks
Hematologic Assessments
- Hemoglobin and ANC were obtained at baseline and at weeks 1, 2, and 4 and then monthly while patients were undergoing therapy and at weeks 4, 12, and 24 after treatment cessation
- Growth factor supplementation and dose-reduction strategies for these patients are outlined in Figure 1
Study End Points
The primary end points were
- Efficacy of erythropoietin versus RBV dose reduction in maintaining hemoglobin >10.5 g/dL
- Efficacy of granulocyte colony-stimulating factor (G-CSF) versus PEG-IFN alfa-2b dose reduction in maintaining ANC >750 cells/mm3
The secondary end points were
- Percentage of patients in whom anemia and neutropenia developed in response to treatment with PEG-IFN alfa-2b and RBV, respectively
- Percentage of patients who achieved an end-of-treatment response (ETR) and an SVR. An ETR was defined as HCV RNA levels <600 IU/mL at the end of treatment
- SVR was defined as HCV RNA levels <600 IU/mL 24 weeks after treatment cessation
Statistics
All analyses were performed as patients were intended to be treated. Parametric statistics were used to evaluate whether baseline characteristics differed between patients. The maximum hemoglobin and ANC decrease was calculated by subtracting the nadir hemoglobin or ANC from the baseline measurement. Similarly, the maximum hemoglobin or ANC increase was calculated by subtracting the highest hemoglobin or ANC from the nadir value.
RESULTS
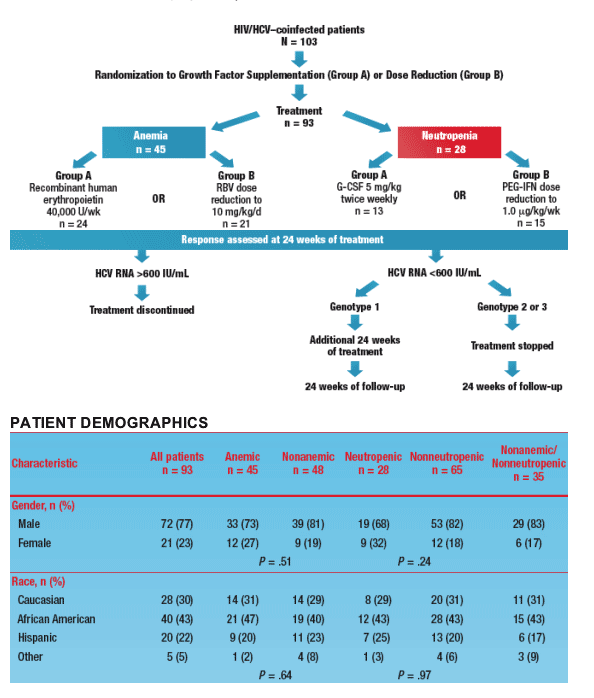
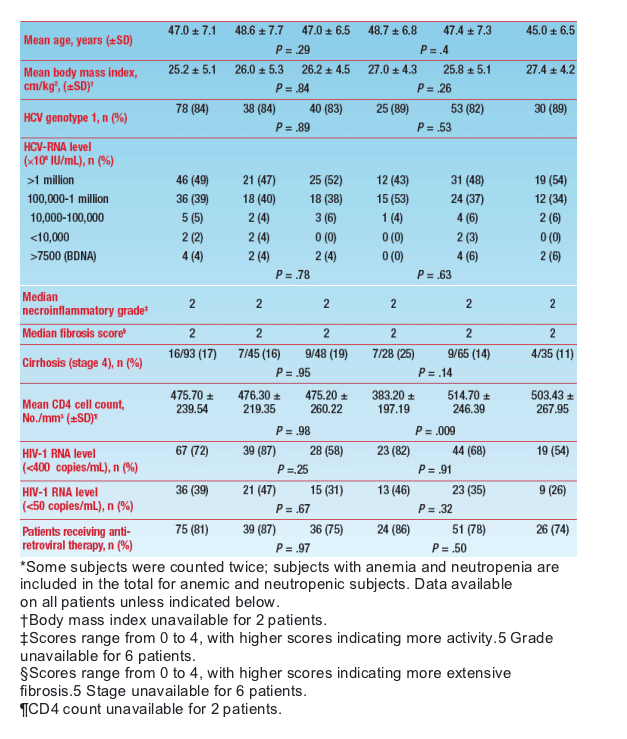
- In total, 103 patients were randomized to treatment and 93 received at least 1 dose of PEG-IFN/RBV (Figure 2)
|
| |
|
 |
 |
|
|