 |
 |
 |
| |
Hi-Dose Consensus IFN in Prior Peg/RBV Users
|
| |
| |
"Successful Treatment With High Dose Consensus Interferon and Ribavirin of Patients With Chronic Hepatitis C Who are Resistant to Peg-Interferon and Ribavirin Therapy"
authors: Kenneth D Rothstein, Ramesh Koka, Angel Fernandez, Holly Hargrove, Shailender Singh, Victor Araya, Santiago J MunozDivision of Hepatology, Albert Einstein Medical Center, Philadelphia, PA
Reported by Jules Levin
DDW, May 2006, Los Angeles
The aim of this study was to evaluate efficacy and safety of high-dose daily CIFN and RBV in HCV patients who failed therapy with PEG-IFN / RBV.
Background provided by investigators:
--The majority of nonresponder and relapser patients with chronic hepatitis C are unable to achieve a sustained virologic response (SVR) with the combination of PEG-Interferon (PEG-IFN) and ribavirin (RBV), especially those who have genotype 1 and advanced disease.
--Consensus interferon (Interferon alfacon-1, CIFN) is a bio-optimized alfa interferon that exhibits increased in-vitro antiviral activity than the naturally occurring alfa interferons 2a and 2b.
--Improved response rates have been reported with high-dose CIFN therapy and RBV for patients who have failed to respond to PEG-IFN / RBV.
(note from Jules Levin: so far we have received study results from single centers conducting relatively small studies in Germany, New Jersey, and other locations. The large phase III study is ongoing & will provide data from a much larger & diverse patient population.
Patients were given 27 ug of CIFN daily and RBV 400 mg BID during the first four weeks. From week 5 to 12, patients received 18 ug of CIFN daily and RBV 400 mg BID daily. At 12 weeks, CIFN was decreased to 15 ug daily while RBV was increased to 1,000-1,200 mg daily for 36 weeks.
Inclusion criteria:
Patients who had been treated with PEG-IFN/ RBV for HCV but did not obtain a SVR were eligible for treatment. Patients who tolerated treatment with PEG-Interferon and Ribavirin. The first ten patients were treated prior to IRB approval.
RESULTS
- Fifty patients have been enrolled in the study
- 72% were male with a mean age of 50 years .
- 96% had genotype 1.
- 72% had stage 3 fibrosis or greater
- 52% had cirrhosis.
- 81% of patients were nonresponders.
- 42% of patients required growth factors : Epoetin alfa 28% Filgrastim 20% Both 8%
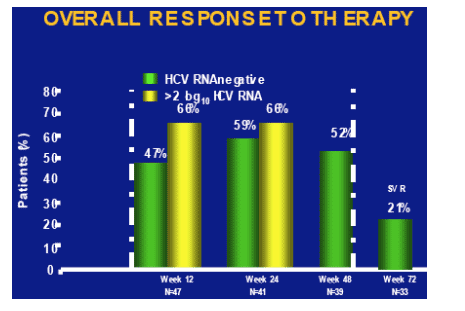
As shown in the table below, at week 72 (n=33) the SVR rate was 21%. After 12 weeks 47% (n=47) were HCV-RNA negative (68% >2 log decrease). At week 24 (n=41) 59% were HCV RNA negative (68% >2 log decrease), and at week 48 (n=39) 52% were HCV RNA negative (end-of-treatment response). So, the relapse rate from 52% to 21% was about 60%.
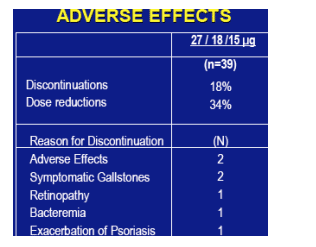
ADVERSE EFFECTS
The most common adverse effects were fatigue, headaches, irritability, weight loss, bone pain and depression in decreasing order of frequency. Rare adverse effects were alopecia and tinnitus. Discontinuations (n=39): 18%. Dose reductions: 34%.
The authors concluded:
This regimen was well tolerated with dose reduction and discontinuation rates similar to other nonresponder trials using PEG-IFN and RBV. For HCV patients with advanced fibrosis who had previously failed therapy with PEG-IFN and RBV, the combination of high-dose CIFN and RBV is an effective option.
|
| |
|
 |
 |
|
|