 |
 |
 |
| |
Consensus IFN for Peg/RBV Nonresponders in Germany
|
| |
| |
"Higher Susceptibility of Peginterferon alfa 2a versus Peginterferon alfa 2b Nonresponder Patients with Chronic Hepatitis C to Retreatment with Consensus Interferon Daily Dosing and Ribavirin"
S. Kaiser, H.G. Hass, B. Lutze, L. Bissinger and M. Gregor
Dept of Medicine, University Hospital of Tuebingen, Germany
Reported by Jules Levin
DDW, May 2006, Los Angeles
Study authors provided this background: Consensus interferon (CIFN) is a bio-optimized interferon with a relatively low half-life requiring daily application for optimal efficacy, however in turn stronger antiviral potency as compared to pegylated interferons as demonstrated for example by the considerable efficacy seen in nonresponders to peginterferon therapy. In this study a subanalysis was perfomed comparing the response rated of pegylated interferon a2a and a2b.
Study Design
The efficacy of CIFN daily dosing and induction therapy followed by CIFN / RBV in PEG IFN combination treatment nonresponders was evaluated. 195 patients have been included, with 90% having genotype 1. Average weight of patients was 76 kg.
Patients were either treated with CIFN at 9 ug QD for 16 weeks or with CIFN 27 ug QD for 4 weeks, followed by 12 weeks of CIFN 18 ug QD. Thereafter, treatment was continued in all treatment groups with CIFN at 9 ug QD with weight-based RBV for 32 - 56 weeks, depending when a patient became first PCR negative, ensuring a treatment period for 48 weeks with a negative PCR.
Demographic Data
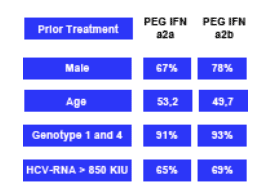
91-93% of patients had genotype 1. 65-69% had HCV RNA >850 KIU. Kaiser did not report important baseline characteristics such as BMI, % with advanced fibrosis & cirrhosis.
RESULTS
Adverse Events
The rates of discontinuation and dose reductions are lower in this study by Kaiser in Germany than in both studies conducted in the USA (Dallas & Philadelphia) & reported at DDW. Although the dose regimens vary as well making it difficult to compare, Kaiser reports overall tolerability for 9 ug regimen was similar to peg/RBV & drop out rates were not different for the two dose regimens in this study; which is why results from a more uniformly conducted phase III should be helpful. I reported the USA study results in the earlier email reports. All reports will be posted Monday on the NATAP website.
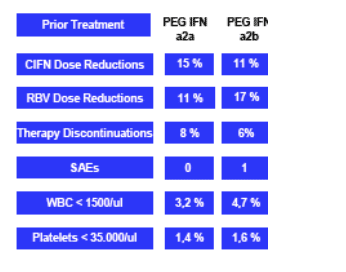
The overall tolerability of the CIFN 9 ug regimen was comparable to a standard therapy with pegylated IFN and RBV, while the CIFN 27/18/9 ug regimen was less tolerable during the high dose induction period. However, drop out
rates were not different between the two dosing regimen. Overall tolerability of the CIFN QD regimen was comparable to a standard therapy with pegylated IFN and RBV, while CIFN even as QD treatment resulted in a lower rate of
thrombocytopenias.
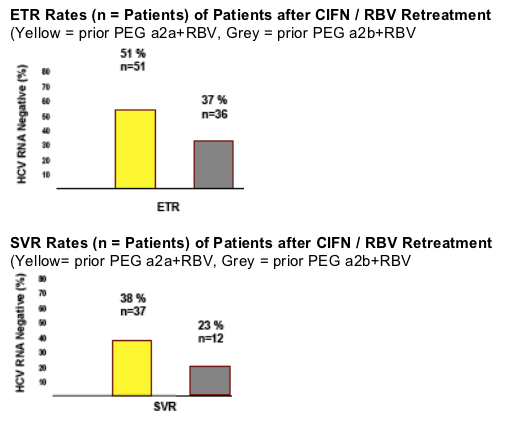
End Of Treatment & SVR Rates
Data show that after the initial 12 weeks of CIFN monotherapy, a primary response with undetectable serum HCV-RNA was observed in 35 % of patients with a prior nonresponse to PEG IFN a2b and in 51 % in prior PEG IFN a2a nonresponders. At the end of treatment, a negative PCR was observed in 37 % in PEG IFN a2b nonrespinders, and in 51% of PEG IFN a2a nonresponders. The sustained viral response rates (SVR) were 21% and 38% for PEG IFN a2b and PEG a2a nonresponders, respectively. Note from Jules Levin: the responses were not reported by dose regimen so Kaiser did not report what the difference in response was between the 9 ug dose regimen & the induction dose regimen. Plus, treatment in this study could be up to 56 weeks depending on when patients became PCR negative.
The authors concluded:
CIFN daily dosing / induction therapy together with subsequent
RBV combination therapy thus shows promising response rates in previous PEG IFN combination therapy nonresponders. Especially PEG IFN a2a nonresponders appear to have a benefit from CIFN QD retreatment. It is concluded that CIFN may be an effective treatment modality for this difficult-to-
treat patient group.
|
| |
|
 |
 |
|
|