 |
 |
 |
| |
Ritonavir Increases levels of Schering HCV Protease Inhibitor SCH503034
|
| |
| |
Reported by Jules Levin
2nd International Workshop on Clinical Pharmacology of Hepatitis Therapy
April 25, 2006
Vienna, Austria
Aside from Schnitzel and Strudel and ice cream, I think the Austrians invented ice cream its so good here, aside from those things the best presentation at the Hepatitis pharmacology Workshop was this one. At the EASL meeting tomorrow Abbott will present the effect of ritonavir boosting on VX-950, the vertex HCV protease inhibitor. It is hard to see how Schering & perhaps Vertex can resist boosting with ritonavir.
- ".....The purpose of the current study was to investigate the in vitro and in vivo interaction between SCH 503034 and ritonavir to determine the potential for pharmackinetic boosting of SCH 503034 by co-dosing with ritonavir..... The metabolism of SCH 503034 was strongly inhibited by ritonavir in both rats and human liver microsomes..... Plasma concentrations of SCH 503034 were strongly enhanced by ritonavir co-dosing.....Pharmacokinetic boosting of SCH 503034 by ritonavir.....should be investigated......Boosting of SCH 503034 may provide increased antiviral activity, more convenient dosing and possibly delayed resistance...."
"Co-Dosing With Rironavir Increases the plasma levels of the HCV Protease Inhibitor SCH 503034" poster 10
Dale Kempf et al. Abbott.
ABSTRACT
Background: Short-term clinical studies using NS3 protease inhibitors HCV (PIs) have demonstrated substantial decreases in HCV plasma RNA. Antiviral activity has been associated with HCV PI plasma trough concentrations: patients with higher plasma exposure experience greater virologic response. Structural similarity with peptidomimetic inhibitors of HIV protease (HIV PIs) suggests that HCV PIs might be substrates for cyrochrome P450 3A (CYP 3A). Co-dosing with ritonavir (RTV), a potent inhibitor of CYP3A, has proven useful for pharmacokinetic enhancement of peptidomimetic HIV PIs, resulting in improved suppression of HIV plasma RNA and more convenient dosing regimens. To determine whether HCV PIs might be candidates for pharmacokinetic boosting we investigated the in vitro and in vivo interaction of the experimental HCV PI SCH 503034 with RTV.
Methods: In vitro rates of metabolism (disappearance of parent compound as assessed by HPLC mass spectral analysis) were measured in pooled human (HLM) and rat (RLM) liver microsomes. Initial concentrations were 1.0 uM SCH 503034 and 0.5 mg/mL microsomes. Rates were estimated in the absence or presence of either 0.4 or 4.0 uM RTV. Male Sprague-dawley rats were dosed with SCH 503034 at 5 mg/kg, orally by gavage. A second set of rats received SCH 503034 (5 mg/kg) along with 5 mg/kg of RTV in the same vehicle.
Results:
In RLM, 38% of SCH 503034 was consumed within 30 minutes (estimated in vitro t1/2 43 min). Metabolism was strongly inhibited by RTV. In the presence of 0.4 4 uM RTV, 100% of SCH 503034 remained after 30 minutes. In HLM, 82% of SCH 503034 was consumed within 30 minutes (estimated in vitro t1/2 13 min.). In the presence of 0.4 uM RTV, only 32% was consumed, and 100% of SCH 503034 remained in the presence of 4 uM RTV. In rats, plasma exposure of SCH 503034 exposure increased by 20-fold with RTV co-dosing. Plasma half-life increased from 0.3 to 2.5 hours.
Conclusions: RTV, at concentrations achieved with doses that enhance HIV PIs, strongly inhibited the metabolism of SCH 503034 in vitro. Co-dosing of peptidomimetic HCV PIs with RTV in humans may substantially increase plasma trough levels, which are associated with virologic response. Pharmacokentic boosting with RTV may be a viable strategy for increasing the efficacy, decreasing the frequency of dosing, and/or lowering the pill burden of HCV PIs.
Background
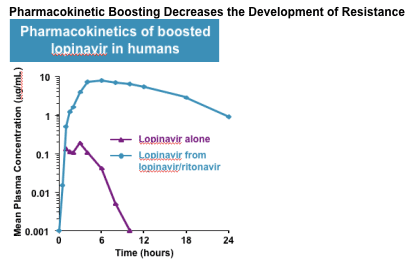
SCH 503034 is a peptidomimetic, mechanism-based HCV NS3 protease inhibitor (PI) in development in combination with pegylated interferon-a (peg-IFN) for the therapy of genotype 1 HCV infection. In vitro, SCH 503034 displays potent, time-dependent inhibition of recombinant single-chain HCV protease in cell-free enzyme assays (overall binding constant 15 nM), and robust inhibvition of the HCV replicon (EC90 400 nM). SCH 503034 has demonstrated dose-related antiviral activity in short-term studies in HCV genotype-1 infected patients. In a two-week monotherapy trial in patients refractory to peg-IFN, the highest dose (400 mg TID) produced a mean maximum HCV viral load reduction of 2.06 log IU/mL. In combination with peg-IFN, this dose produed a mean 2.9 log IU/mL viral load decline. Importantly, a positive correlation between decline in viral load and plasma trough concentrations of SCH 503034 was observed suggesting that trough levels may be predictive of virologic response. In a single-dose pharmacokinetic study in healthy subjects, the 400 mg dose of SCH 503034, the 400 mg dose of SCH 503034 produced plasma concentrations that barely exceeded the in vitro EC90, followed by relatively rapid clearance.
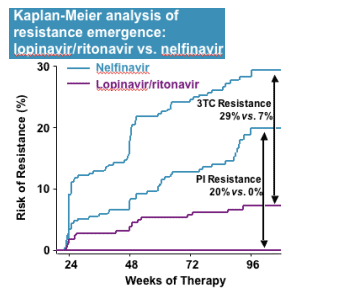
The route(s) of metabolic clearance of SCH 503034 have not been reported. However, its relatively large, hydrophobic, peptidomimetic structure resembles the structures of various peptidomimetic inhibitors of HIV protease, the efficacy of which is also correlated to plasma trough concentrations. HIV PIs are largely or exclusively metabolized in both humans and animal models through ixidation by the 3A isozyme of cytochrome p450 (CYP 3A). One HIV PI, ritonavir, is also a potent CYP 3A inhibitor, and has been shown to block the metabolism of other PIs and substantially enhance their plasma levels. In the treatment of HIV disease, the plasma concentrations of HIV protease inhibitors are commonly "boosted" by co-dosing (or in one case co-formulation) with ritonavir, providing improved efficacy, more convenient dosing, and a delay in the development of drug resistance.
The purpose of the current study was to investigate the in vitro and in vivo interaction between SCH 503034 and ritonavir to determine the potential for pharmackinetic boosting of SCH 503034 by co-dosing with ritonavir.
METHODS
SCH 503034 was synthesized according to literature methods.
In vitro Metabolism
- Rat liver microsomes were harvested from male Sprague-Dawley rats.
- Pooled human liver microsomes (n=10 individuals) were used.
- The disappearance of parent SCH 503034 at 1 uM initial concentration was monitored over 30 minutes in triplicate.
- Inhibition by 0.4 and 4.0 uM ritonavir was assessed.
Pharmacokinetic Studies
- SCH 503034 and ritonavir were prepared as solutions in PEG-400.
- Male Sprague-Dawley rats were dosed orally with SCH 503034 by gavage at 5 mg/kg, alone or with 5 mg/kg co-dose of ritonavir.
RESULTS
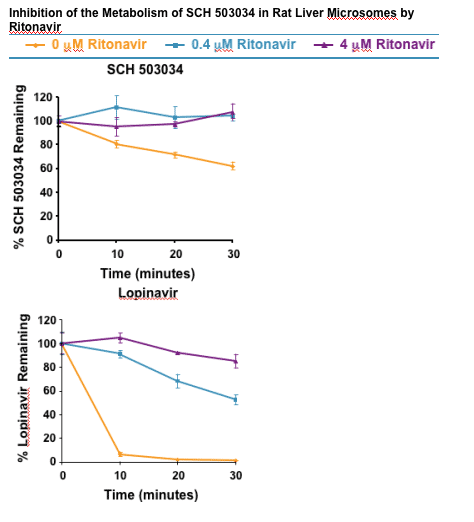
The metabolism of SCH 503034 was strongly inhibited by ritonavir in both rats and human liver microsomes.
- In rat and human liver microsomes, 38% and 82%, respectively, of SCH 503034 was metabolized in 30 minutes.
- In the presence of 0.4 or 4.0 uM ritonavir, the in vitro metabolism of SCH 503034 was 100% inhibited in rat liver microsomes.
- In the presence of 0.4 uM or 4.0 uM ritonavir, the in vitro metabolism of SCH 503034 was 72% and 100% inhibited in human liver microsomes, respectively.
- The HIV PI lopinavir, which is substantially boosted by ritonavir in vivo, was used as a positive control.
- In both rat and human liver microsomes, >90% metabolism of lopinavir was observed within 10 minutes.
- The in vitro metabolism of lopinavir was substantially inhibited at both 0.4 and 4.0 uM ritonavir.
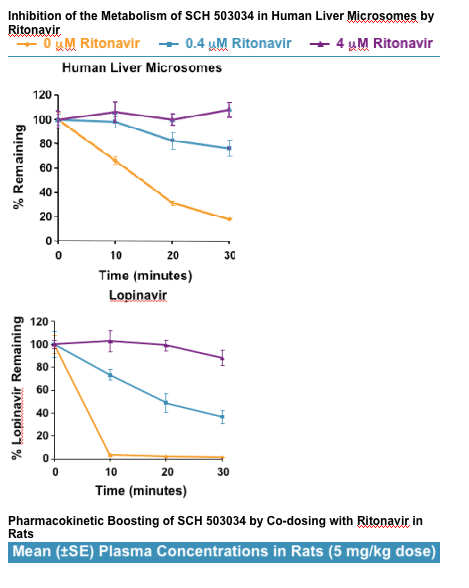
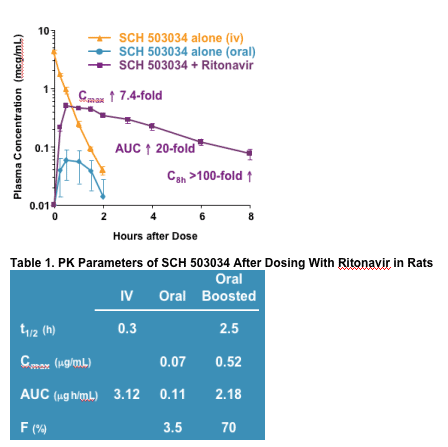
Plasma concentrations of SCH 503034 were strongly enhanced by ritonavir co-dosing.
- In rats, SCH 503034 exhibited high clearance and low bioavailability.
- Co-dosing with ritonavir increased the plasma exposure of SCH 503034 by >20-fold. The Cmax increased by >7-fold.
- Whereas plasma levels of SCH 503034 declined to unquantifiable with <3 hours when dosed orally alone, the oral combination of SCH 503034/ritonavir produced sustainable concentrations of SCH 503034 >100 ng/mL for >6 hours. SCH 503034 t1/2 increased by nearly 10-fold.
- DISCUSSION
The metabolism of SCH 503034 is strongly inhibited by ritonavir both in vitro and in vivo. SCH 503034 metabolism appears to be primarily due to CYP 3A. Since phase 2 metabolism is relatively unlikely for SCH 503034 based on its structure, pharmacokinetic enhancement of SCH 503034 by co-dosing with ritonavir in humans is likely.
- The current highest dose of SCH 503034 (400 mg TID) may produce plasma concentrations that achieve suboptimal antiviral activity (note from Jules Levin: a new dosing arm of 800 mg TID of SCH 503034 was recently added to the phase II study). Co-dosing with ritonavir may allow for increased efficacy of SCH 503034.
- The low doses of ritonavir required for pharmacokinetic boosting of HIV PIs (100-200 mg/day) produce ritonavir exposures only 5-8% that of gull-dose (600 mg BID) ritonavir. Boosted HIV PIs are well-tolerated in HCV-HIV coinfected individuals, with no evidence of incremental hepatoxtoxicity in several studies comparing ritonavir-boosted to non-boosted HIV PI-based regimens.
CONCLUSIONS
- Pharmacokinetic boosting of SCH 503034 by ritonavir in humans is likely, and should be investigated.
- Boosting of SCH 503034 may provide increased antiviral activity, more convenient dosing and possibly delayed resistance.
- Boosting doses of ritonavir are safe and well-tolerated in HCV-infected patients.
- Pharmacokinetic boosting by ritonavir may be useful for other HCV protease inhibitors.
|
| |
|
 |
 |
|
|