 |
 |
 |
| |
NM283, HCV Polymerase Inhibitor Study in Naives: dose change for development
|
| |
| |
Reported by Jules Levin
EASL, April 29, 2006, Vienna, Austria
"Early Clearance of HCV RNA with Valopicitabine (NM283) plus Peg-Interferon in Treatment-Naive Patients with HCV-1 infection: First Results from a Phase IIb Trial"
Doug Dieterich reported the study results for the Valopicitabine 006 Study Group.
Protocol Amendment: Dose Modification
Valopicitabine (NM283) Phase IIb Study in Treatment Naive Patients
Due to GI tolerance issues for some patients at 800 mg/d NM283 dose level, a dose reduction has been implemented for development of NM283:
Patients receiving 800 mg NM283/pegIFN will continue treatment at reduced dose, randomly assigned (1:1) to:
- 200 mg valopicitabine/peg-IFN_ or
- 400 mg valopicitabine/peg-IFN_
12 patients with HCV RNA ≥ 600 IU/mL at time of protocol amendment; all discontinued treatment
Patients in Group B in this study (200mg NM283/pegIFN) are contining study treatment unchanged.
New Drugs & Treatment Paradigms for Hepatitis C
Small molecule anti-viral compounds are breaking the old paradigm of HCV treatment
Future anti-HCV regimens will involve drugs with different mechanisms of action
There is still much to learn:
- Synergy of antiviral effects
- Resistance development and avoidance
- Toxicities of drugs in the antiviral classes: nucleoside and non-nucleoside polymerase inhibitors; protease inhibitors; potentially others
Valopicitabine (NM283) is the first nucleoside-type HCV Pol inhibitor, now in Phase IIb clinical trials:
- Oral agent
- Plasma half life (4-6 hrs) & intracellular half life (15 hrs) support once daily dosing
Valopicitabine (NM283)
Key Clinical Findings To Date
(All in HCV-1 patients)
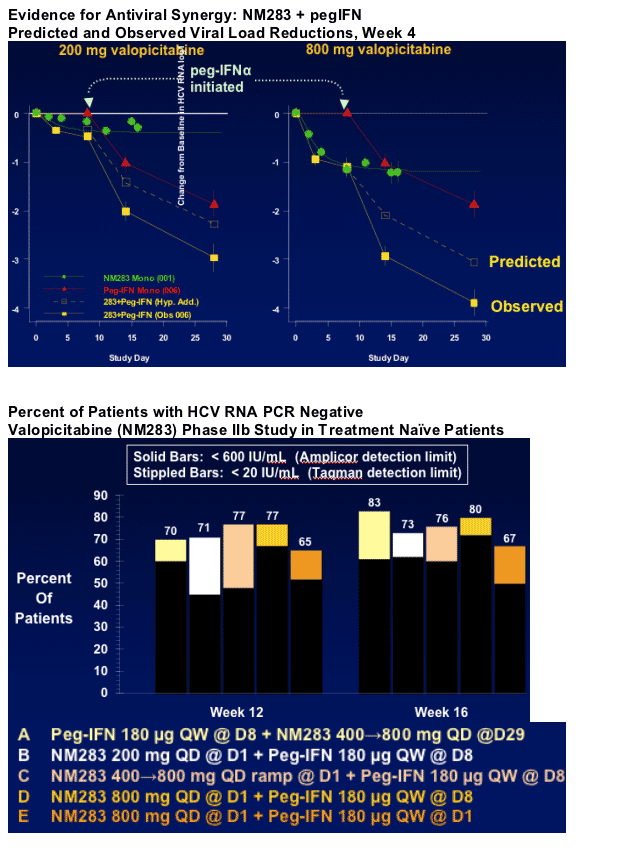
Dose-related HCV RNA reductions with valopicitabine monotherapy1
Greater HCV RNA reduction with valopicitabine plus pegIFNa-2b2
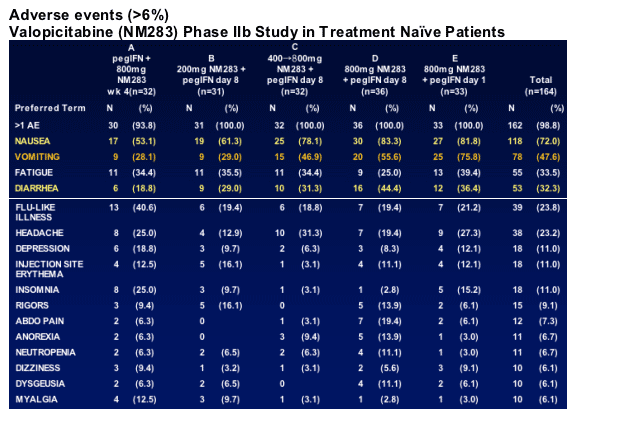
Dose-related GI side effects1, 2, 3
- Nausea, vomiting, occasional diarrhea, primarily at doses ≥400 mg/d
- Increased with the addition of pegIFN
- Usually mild-moderate, transient, and manageable 1,2,3
- No hematologic side effects for valopicitabine monotherapy1, 2, 3
1Afdhal AASLD 2004; 2Rodriguez-Torres DDW 2005; 3O'Brien AASLD2005
Objectives
Valopicitabine (NM283) Phase IIb Study in Treatment Naive Patients
Overall purpose: to identify optimal dosing regimens for further investigation Phase III studies
Efficacy objectives:
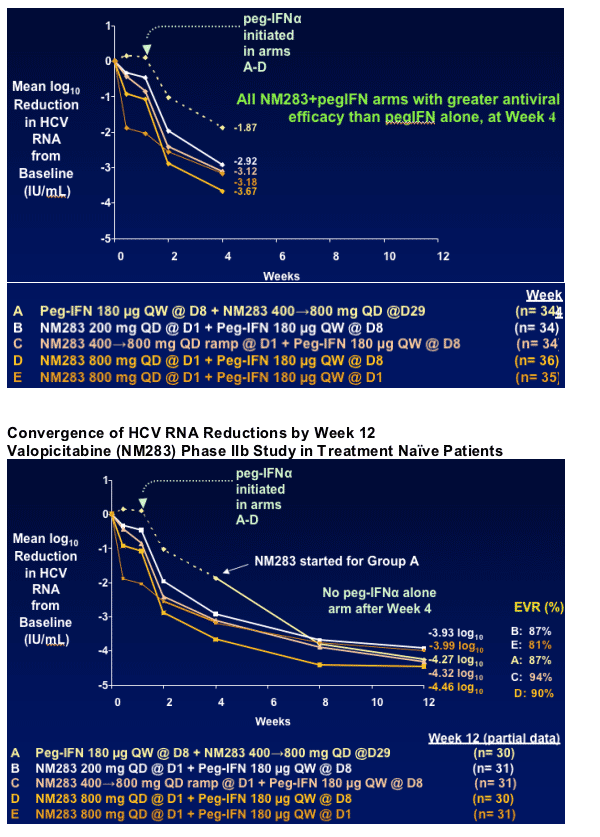
- NM283 dose-related antiviral efficacy (HCV RNA reductions) for 4 different NM283/pegIFN_ regimens
- Compare antiviral efficacy vs pegIFN alone at Week 4
Eventual assessment of SVR rates for the 5 regimens
Safety/tolerance objectives
- NM283 dose-related safety/tolerance
Standard safety data: SAEs, AEs, labs, discontinuations
Key Eligibility Criteria
Valopicitabine (NM283) Phase IIb Study in Treatment Naive Patients
- 18-65 years of age, male or female
- HCV genotype 1
- Treatment Naive
- no previous antiviral therapy for HCV
- Baseline
- HCV RNA ≥5 log10 IU/mL
- ALT >1.0 x ULN and <5 x ULN
- Compensated liver disease
- Candidate for interferon therapy
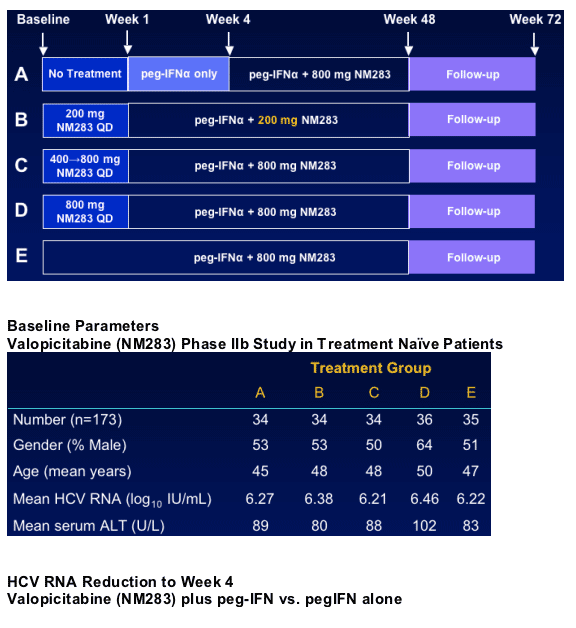
Initial Study Design
Valopicitabine (NM283) Phase IIb Study in Treatment Naive Patients
Safety Summary
Valopicitabine (NM283) Phase IIb Study in Treatment Naive Patients
GI side effects common with initial dosing, usually mild-moderate; lessen within 1-2 weeks in 70-80% of affected patients, typically manageable with continued treatment
32 of 173 (18%) patients discontinued by Week 12
- 24 (14%) for adverse events, mostly for GI side effects
- only 2 patients in 200 mg cohort discontinued
8 SAEs reported by Week 12 (all in 800 mg dose groups)
- 2 attributed to NM283 or NM283+pegIFN: dehydration with renal insufficiency and pancreatitis; hyponatremia/hypokalemia
- 6 non-attributable: eye infection, flu with dehydration, CHF/diabetes, chest pain, numbness in arm/confusion, burn
- All patients recovered
Grade 3/4 lab abnormalities
- Most were attributable to peg-IFN_ (øWBC, øANC, øplatelets)
- 9 patients with Grade 3/4 AST and 2 patients with Grade 3/4 lipase elevation, all in 800 mg treatment groups
FUTURE DIRECTIONS
200-400 valopicitabine doses chosen for further study in treatment-naive patients. Good antiviral efficacy with good safety/tolerance. Ribavirin / NM283 interaction study - starts 2Q2006. Potential investigation of double and triple regimens (NM283 + pegIFN + ribavirin) in phase III clinical trials
|
| |
|
 |
 |
|
|