 |
 |
 |
| |
VX-950 HCV Protease Inhibitor Resistance Profile
|
| |
| |
"Wild-Type HCV NS3 Protease Re-emerges during Follow-up After 14 Days of Dosing with VX-950 in Patients with Genotype 1 HCV"
Tara Kieffer from Vertex reported this study data at EASL Vienna, Austria, April 27, 2006
BACKGROUND provided by Kieffer.
HCV replication creates sequence diversity in viral genomues generating a population of viral quasispecies;
- high levels of HCV replication
- error prone RNA-dependent RNA polymerase
Variants conferring various levels of resistance to a direct acting antiviral may exist at low frequency prior to drug treatment.
Treatment with a specifically-targeted antiviral drug selects for drug resistant viral variants (e.g. HIV, HBV)
- strong suppression of wild-type virus is required for emergence of these variants.
Author Summary
VX-950 is an oral HCV protease inhibitor that has demonstrated rapid and dramatic antiviral activity. Mutations conferring various levels of resistance are selected in some patients during dosing with VX-950 alone: emergence of resistant virus underlies breakthrough (HCV RNA return) and plateau response). High-level resistant variants are rapidly replaced by wild-type in the absence of VX-950 selective pressure: the rate at which resistant variants are replaced by wild-type virus after dosing may be due to their impaired fitness. At DDW in May 2006 in LA, Vertex is presenting results of 28-day study of triple combination of VX-950 plus Pegasys and ribavirin.
Kieffer said - Can we prevent the selection of resistance mutations? Kieffer then said VX-950 in combination with pegIFN may prevent selection of resistant variants associated with viral breakthrough. The results of the combination VX-950 + Pegasys 14-day study were presented. Antiviral results after 14 days of dosing showed a median 5.5 log10 reduction in HCV RNA in patients receiving VX-950 and peg-IFN; six of eight patients had viral levels below the limit of quantitation (30 IU/mL) at 14 days, and four of eight also achieved viral levels below the limit of detection (10 IU/mL). All patients completed dosing, and no serious adverse events were reported. All adverse events in the group who received VX-950 were mild.
Following the completion of this study, patients were offered a full course of treatment with peg-IFN and ribavirin.
All eight patients in the combination group opted to receive treatment with peg-IFN and ribavirin. After three months, all eight patients were shown to have undetectable levels of HCV RNA. Resistance testing is ongoing.
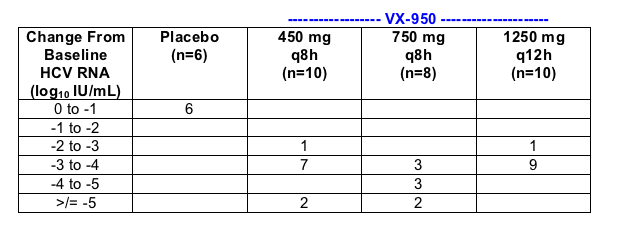
VX-950 Phase 1b, 14-day Multiple Dose Study
VX05-950-101 demonstrated a rapid and dramatic reduction in plasma HCV RNA in all 28 patients dosed with VX-950
- chronically infected with genotype 1 hepatitis C virus
- dosed with protease inhibitor VX-950 for 14 days
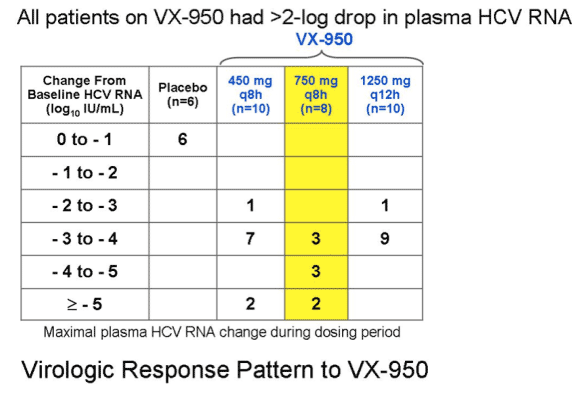
- 450 mg q8h [every 8 hours] (n=10)
- 750 mg q8h (n=8)
- 1250 mg q12h (n=10)
- placebo (n=6)
Optimal dose group (750 mg q8h) induces about 3 log10 drop during the first 24 hours of dosing and had a median 4.4 log drop in plasma HCV RNA after 14 days of dosing.
All Patients on VX-950 had >2-log Drop in Plasma HCV-RNA
Characterization of Viral Variants During and After VX-950 Dosing
The goal of this sub-study:
-- To assess whether variants with decreased drug sensitivity were selected in patients dosed with VX-950 alone.
-- To determine whther variants persisted after the end of the VX-950 14-day dosing period.
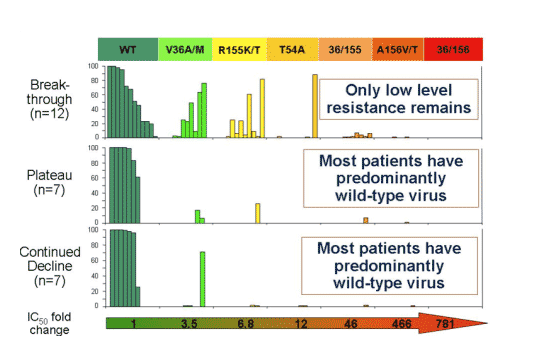
Virologic Response Pattern
1. 14-day dosing period
2. post-dosing, day 14 to 28: there was a slow return of HCV RNA
3. Long-term follow-up: 3 to 7 months
Patients were characterized as in these groups based on viral response and resistant was characterized for each group:
--Placebo (n=6)
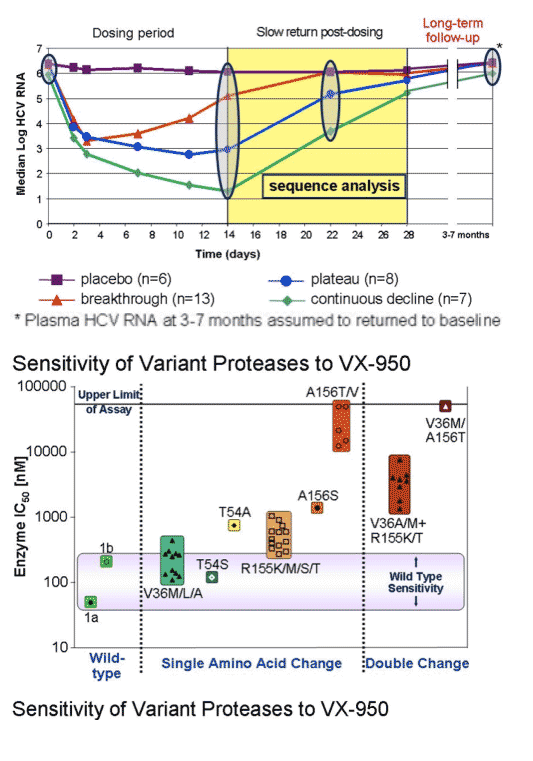
--Breakthrough (n=13): these patients had median viral load reductions of about 3 logs from baseline of about 6 logs to just over 3 logs, the reduction peaked at day 3 & then viral load (HCV RNA) started to return towards baseline & by day 14 was almost at baseline. By day 22 HCV RNA returned to baseline, about 6 logs.
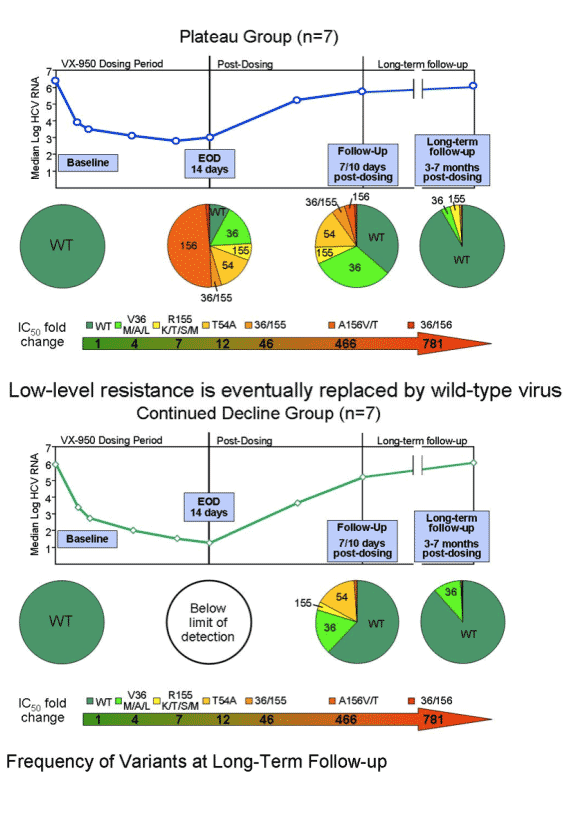
-- plateau (n=8): these patients also had about a 3-log reduction in viral load at day 3 from about 6 logs down to about 3 logs. But instead of returning towards baseline viral load plateaued for the remainder of the 14 day study. At day 14 viral load decline was the same from 6 to 3 logs. From day 14 to day 28 HCV RNA slowly returned to baseline, more slowly than for breakthrough patients.
--Continuous decline (n=7): these patients had the greatest decline in HCV RNA. Baseline was also about 6 logs, at day 3 viral load was about 3 logs. But HCV RNA continued to decline so viral load was at 2 logs by day 8, at 1.5 logs at day 10, and 1.25 logs at day 14, a continuous steady decline. From days 14-28, HCV RNA increased slowly and almost reached baseline but not quite at day 28.
High Sensitivity Method For Characterization of Viral variants During Antiviral Therapy
Cloning and sequencing of a mean of 80 clones per patient per time point was performed. Analysis of HCV NS3 protease was performed on amino acids 1-181)
-baseline
-end of dosing: day 14 (EOD)
-short-term follow-up: day 21-24 (FU)
-long-term follow-up: 3-7 months (LTFU)
Assay detects variants present at about 5% frequency.
Process
--NS3 protease was taken from patient clones
--Expression & purification
--evaluate VX-950 IC50 in HCV protease enzyme assay
--clone mutants into HCV 1b strain background
--evaluate VX-950 IC50 in HCV replicon assay
RESULTS
Senstivity of Variant Proteases to VX-950
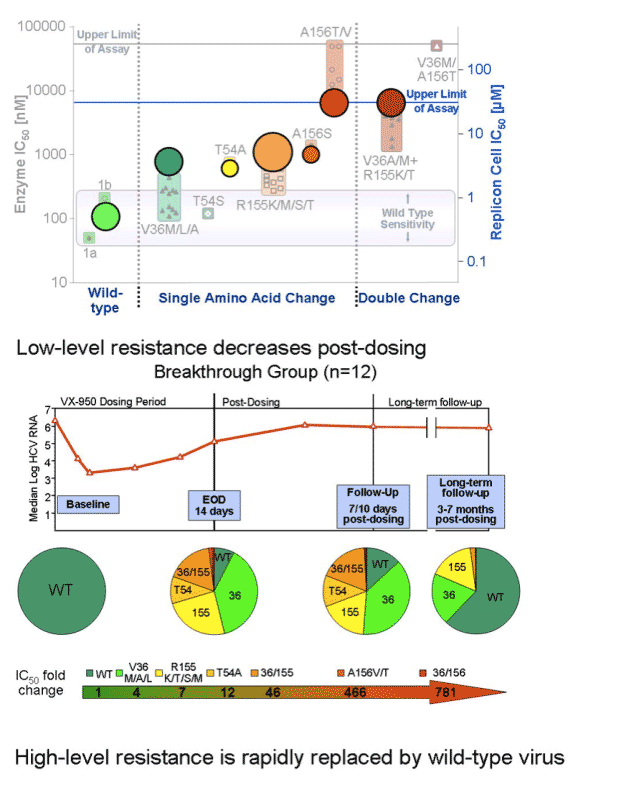
HCV was wild-type virus at baseline. Several days into therapy the V36/M/A.L mutation emerged (IC50 fold-change: 4), then the R155/K/T/S/M (IC50 fold-change: 7), than the T54A (IC50 fold-change: 12). After the end of dosing (EOD) the double mutant 36/155 emerged (IC50 fold-change: 46), followed by A156V/T (IC50 fold-change: 466), then the 36/156 (IC50 fold-change: 781). There was little wild-type virus during this time sequence but the percent of virus that was wild-type increased over time. In the breakthrough patients the 155 mutation and the 36 mutation were more prevalent. In the plateau patients the 156 mutation was more prevalent, but the others were present. In these patients over time the 156 became less prevalent & the 36 was more prevalent; percent of wild-type virus increased. In the breakthrough patients by LTFU the percent of wild-type returned to about 60%, and the 155 & the 36 mutations were still equally present. In the plateau patients during LTFU the percent of wild-type returned to about 90% and the 36 & 155 mutations were present at low levels. In the continuing decline patient group at EOD Vertex reported virus was "below the level of detection"; durinf post-dosing follow-up mutations at 155, 54, and 36 were present, and wild-type was about 60%. By LTFU, wild-type returned to about 90% but 36 mutation was detected.
During this time sequence both replicon cell and enzyme IC50s increased in parallel. IC50s continued to increase throughout the time sequence.
|
| |
|
 |
 |
|
|