 |
 |
 |
| |
The HCV Protease Inhibitor SCH 503034 in Combination with PEG-IFN a-2b in the treatment of HCV-1 PEG-IFN +/- Ribavirin Non-Responders: Antiviral Activity & HCV Variant Analysis
|
| |
| |
Reported by Jules Levin
EASL, April 26-30, 2006
Vienna, Austria
S Zeuzem (Saarland University Hospital, Homburg, Germany) reported these study results for his coinvestigators and Schering-Plough at EASL in an oral presentation. Study sponsored by Schering-Plough Research Institute.
At the Schering-Plough symposium it was reported that in treatment-naive patients a median 1-log viral load reduction was observed in a short-term study, I think 14-days monotherapy.
Background provided by Zeuzem: Evasion of Intracellular Host Defense by HCV
Hepatitis C virus / host interaction with-
- RIG-1/cardif
- TLR-3/trif
serves to control host immune responses and may attenuate the action of IFN.
This interaction therefore provides a foundation for persistent HCV replication and spread.
NS3 protease may represent a dual therapeutic target.
HCV protease inhibitors will
-Suppress viral replication
-improve host interferon responsiveness
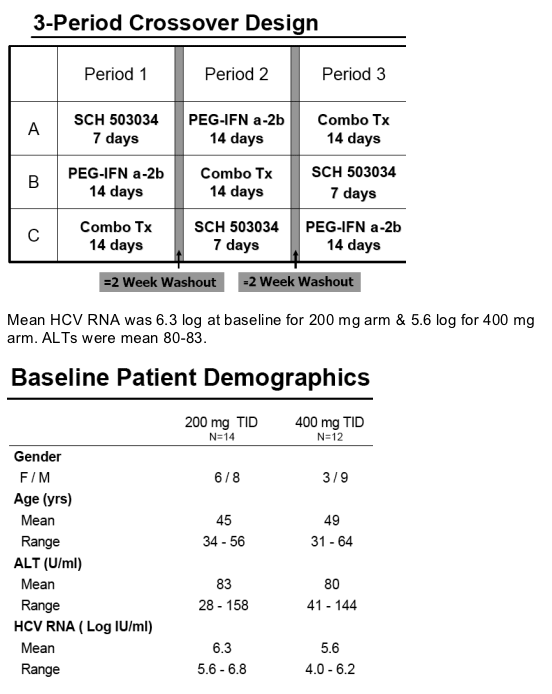
SCH 503034 + Peg-IFNa-2b in HCV-1 Non-Responders: study design
Population:
--HCV-1 Peg-IFN a-2b nonresponders: previous treatment was for 12 weeks with or without ribavirin ; HCV RNA reduction was <2 logs. Patients have compensated liver disease for this study.
Study Design:
Open-label, 3-period crossover
All patients received all 3 regimens with at least a 2 week wash-out period:
--Peg-IFN a-2b: 1.5 ug/kg once weekly (QW)
--SCH 503034: 200 mg or 400 mg TID (3 times a day)
--Combination of the Peg-IFN a-2b (1.5 ug/kg QW) and SCH 503034 (200 mg or 400 mg TID)
METHODS
Pharmacodynamics
-SCH 503034 Monotherapy:
days -1, 1-4, 6, 7, and D28 (F/U)
-Peg-IFN alfa-2b +/- SCH 503034
days -1, 1.4, 6, 8-11, 13-14 and Day 28 (F/U)
Resistance evaluation
--Day 1, 6 PI-monotherapy
--Day 1, 6, 13 Peg-IFN alfa 2b +/- SCH 503034
--Day 28 F/U final period
--cDNA sequencing
--sensitivity about 10-20%
Safety
--vital signs
--clinical lab tests
--time-matched ECG
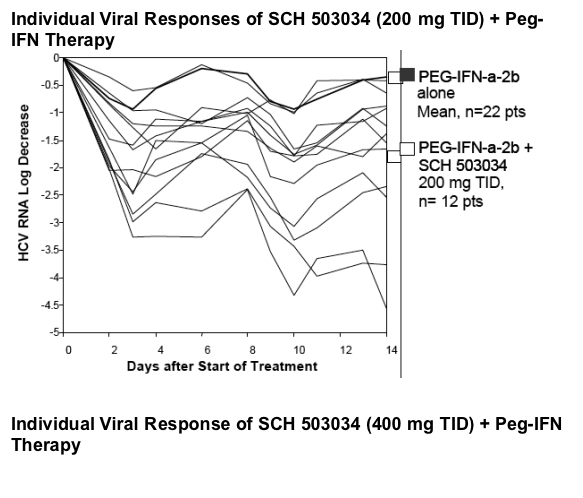
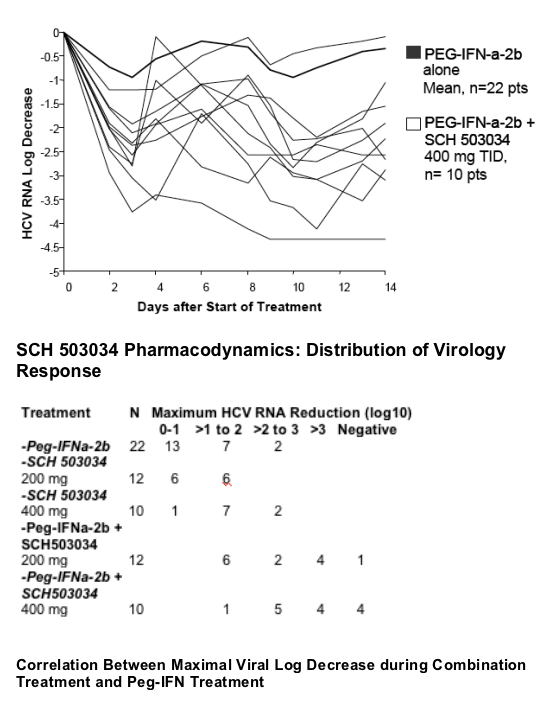
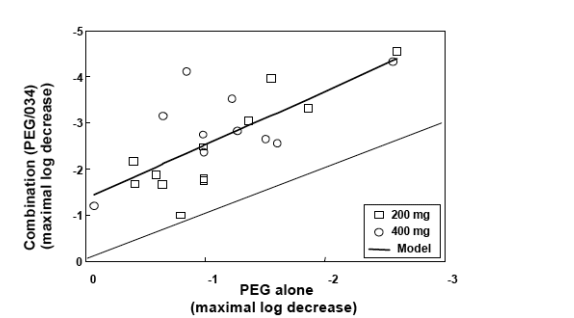
The slope is in line with in vitro data that shows synergistic activity between PegIFN and SCH503034.
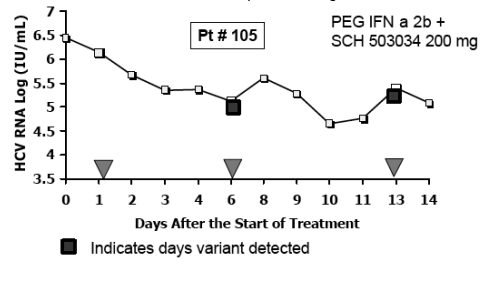
Sequence Analysis of HCV Protease
-- 18/19 patients had no detectable variants
-- patient #105: a single mutation at position T54 was identified during SCH 503034 mono- and combination tx
-- Variant became non-detectable after washout period
-- Quasi-species analysis are under way
NOTE from Jules Levin: at the meeting after Zeuzem gave his talk I asked him from the microphone why they had so little resistance, 1 mutation in 1 patient, compared to VX-950, for which Vertex presented much resistance data, the response Zeuzem said was-the resistance test used in this study was not as sensitive and SCH503034 was not potent enough to cause resistance.
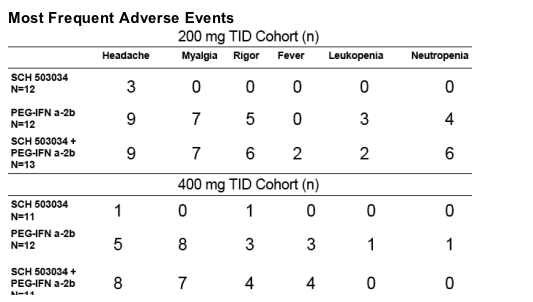
Safety
--Sch503034 + PegIFNa-2b combination therapy was well tolerated.
--Mosr AEs were mild and moderate
--No dose-related increase in AE frequency
--Most frequently reported AEs in combination therapy were: headache, myalgia, fever
--Single SAE leading to discontinuation (seizure)
--Clinical lab values and ECGs were similar to Peg-IFNa-2b alone.
AUTHOR SUMMARY
SCH 503034 + PegIFNa-2b was well tolerated in genotype 1 patients who had previously not responded to standard therapy.
Combination did not affect the oscillatory pharmacodynamic pattern of PegIFNa-2b.
Antiviral activity of SCH 503034+ PegIFNa-2b was potent and at least additive (combination superior to either monotherapy).
4/10 HCV-1 non-responders became HCV RNA negative within two weeks of SCH 503034 (400 mg TID) + PegIFNa-2b.
|
| |
|
 |
 |
|
|