 |
 |
 |
| |
5 Year Followup of SVRs with PegIntron/RBV-98%
Continued SVR5
|
| |
| |
Reported by Jules Levin
EASL, April 2006, Vienna, Austria
....of 492 sustained responders....Only 5 patients in the SR group relapsed in the 5 year follow-up. All relapsed within the first year..... The Kaplan-Meier estimate for continued sustained response over 5 years was 97-99+/-1%.....
"Sustained Virologic Response (SVR) to Interferon alfa-2b +/- Ribavirin Therapy at 6 Months Reliably Predicts Long-term Clearance of HCV at 5-Year Follow Up"
J.G. McHutchison1, M.L. Shiffman2, S.C. Gordon3, K.L. Lindsay4, T. Morgan5, G. Norkrans6, R. Esteban-Mur7, T. Poynard8, P.J. Pockros9, J.K. Albrecht8, C. Brass10
1Division of Gastroenterology and DCRI, Duke University, Durham, NC; 2Hepatology Section, VCU Medical Center, Richmond, VA; 3Division of Hepatology, Henry Ford Hospital, Detroit, MI; 4USC Keck School of Medicine, Division of Gastroenterology, Los Angeles, CA;
5VA Medical Center, Department of Medicine/Gastroenterology, Long Beach, CA, USA; 6Department of Infectious Diseases, Goteborg University, Sweden; 7Department of Hepatology, Hospital Vall d'Hebron, Barcelona, Spain;
8Service d'Hepato-Gastroenterologie, Groupe Hospitalier Pitie-Salpetriere, Universite Paris VI, Paris, France; 9Division of Gastroenterology, Scripps Clinic, La Jolla, CA, USA; 10Schering-Plough Research Institute, Kenilworth, NJ, USA
ABSTRACT
Background and Aims: Absence of detectable serum HCV-RNA six months after treatment defines SVR. Data beyond six months is limited. Primary aim was to determine long-term SVR and clinical outcomes in patients treated with interferon-alfa-2b+/-ribavirin.
Methods: 1071 patients treated with interferon-alfa-2b+/- ribavirin from six clinical trials were followed up to five years with annual measures of hematology, biochemistry, GI/liver examination and quantification of HCV RNA by PCR with sensitivity of 100 copies/ml (National Genetics Institute) and after 3/01 by TaqMan (Schering-Plough: sensitivity of 29IU/ml).
Results: 492 sustained responders (SR) and 579 non-responders (NR) entered the trial and were followed for a mean of 283 and 131 weeks, respectively, after the standard 24 week follow-up period. 61% of SR (302) and 28% of NR (160) patients completed at least five years of follow-up. Only 5 patients in the SR group relapsed in the 5 year follow-up. All relapsed within the first year and had elevated ALT and >4 log increase in viral load. The Kaplan-Meier estimate for continued sustained response over 5 years was 99+/-1%. Progression of liver disease occurred in 2 patients in the SR and 5 in the NR group. 3 SR and 8 NR patients died during the follow-up period. Only 2 deaths were related to liver disease progression (HCC). Only 2 of 71 SAEs was 'possibly' related to interferon/ribavirin therapy.
13 SR and 5 NR patients had a liver biopsy performed >5 years with hepatic HCV RNA testing in ten. Of this group, all 8 SR patients were negative for hepatic HCV RNA while the two NR patients were positive. Posttreatment
values of hepatic activity (Knodell I+II+III) increased in the 5 NR patients (mean 6.0 pre-Tx to 5.0 post-Tx to 7.8 FU) undergoing liver biopsy while the 12 SR
patients remaining HCV RNA negative showed little change.(8.8 to 3.0 to 2.7).
Conclusions: This large study confirms that sustained loss of serum HCV RNA 6 months following the completion of interferon-alfa-2b +/-ribavirin treatment is an excellent predictor of long-term clearance of the virus from the serum and may predict clearance of hepatic infection and maintenance of histological improvement.
AUTHOR CONCLUSIONS
- This large study, consisting of more than 1000 patients, confirms that a sustained virological response (loss of serum HCV RNA 6 months after completion of interferon alfa-2b with or without ribavirin therapy), regardless of treatment regimen, is maintained for 5 years in most patients
- Hence, SVR 24 weeks after cessation of therapy is an excellent predictor of long-term clearance of the virus from the serum
- Patients treated with interferon alfa-2b with or without ribavirin who achieve SVR have a 97% to 99% probability of remaining virus free 5 years later
- SVR may predict clearance of hepatic infection and maintenance of histological improvement
- These findings confirm the concept of 'cure' in that these patients, despite close follow-up, have essentially no evidence of clinical or virological disease
- The risk for late-occurring adverse events from interferon-alfa-2b with or without ribavirin treatment is minimal
METHODS & STUDY POPULATION
Patients
- 1071 treatment-naive patients or patients with relapsed chronic hepatitis C who were treated with interferon alfa-2b (Intron A) +/- ribavirin (Rebetol) and completed 24 weeks of follow-up were recruited from six earlier clinical trials (treatment naive: C95-132, I95-143, C96-114; relapse: C95-144, I95-145, C96-318) that involved 69 US and international centres. Informed consent was obtained from all patients
Assessments
- Measures of hematology, biochemistry, gastrointestinal/liver examination, and quantification of HCV RNA by polymerase chain reaction (PCR) with sensitivity of 100 copies/ml (National Genetics Institute) and after March 2001 by TaqMan (Schering-Plough: sensitivity of 29 IU/ml) were performed yearly during the 5-year post-treatment follow-up
- Clinical details regarding liver disease progression were collected each year in the form of a simple case report
- SVR was defined as <100 copies of HCV RNA/ml serum (Superquant; National Genetics Institute) or <29 IU/ml (TaqMan)
- Patients who had persistent HCV RNA negativity at 5 years of follow-up were then asked to undergo liver biopsy
RESULTS
Patient Flow
Of the 1071 patients enrolled in the long-term follow-up study, 492 had achieved an SVR and 579 had not achieved an SVR (nonresponders; NRs) during the previous trials
- Of the 492 SVR patients, 302 (61%) completed at least 5 years of follow-up with a median duration of 283 weeks
- Of the 579 NRs, 160 (28%) completed at least 5 years of follow-up with a median duration of 131 weeks
- Only five of the 492 SVR patients had definitive relapses during follow-up
- Definitive relapse was defined as HCV RNA >100 copies/ml, >4 log10 increase in viral load, and abnormal ALT ratio at any time during long-term follow-up
- Seven patients were considered to have possible disease relapse during follow-up; these patients had inconsistent HCV RNA findings during follow-up
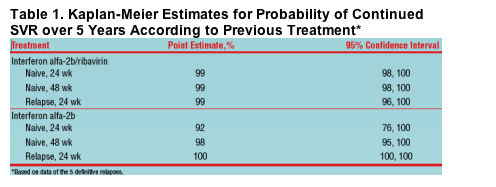
Probability of SVR for the 5-Year Post-Treatment Follow-Up
- Kaplan-Meier estimate of continued SVR over 5 years was 99% (95% CI: 98%, 100%) based on the 5 definitive relapsers
- The estimate was 97% (95% CI: 95%, 98%) if the 7 possible relapsers were included in the calculations
The probability of maintaining SVR over 5 years was high, regardless of previous treatment (monotherapy or combination therapy) or treatment status (interferon alfa 2b-naive; interferon alfa-2b relapser) was high (based on the 5 definitive relapsers)
- Similar curves were obtained when the 7 possible relapsers were included in the calculations
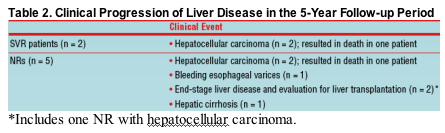
Progression of Liver Disease in Subjects Treated with Interferon alfa-2b with or without Ribavirin
- Seven patients-2 SVR patients and 5 NRs-had clear clinical signs of liver disease progression during long-term follow-up
- Seriously progressive liver disease occurred less frequently among SVR patients than among NRs
- Only 2 deaths were related to liver disease progression (secondary to hepatocellular carcinoma)
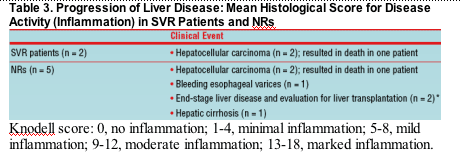
- Of 18 patients (13 SVR, 5 NR) who underwent liver biopsy at >5 years, 10 (8 SVR patients, 2 NRs) were available for hepatic HCV RNA testing. All SVR patients were negative (<100 copies/ml at >5 years of follow-up) for hepatic HCV RNA, whereas all NRs were positive (3670,000 copies/ml at >5 years of follow-up)
- Post-treatment hepatic activity (inflammation) increased in NRs, whereas SVR patients showed improvement during long-term follow-up (table 3)
- No SVR patients had increased Kodell or Metavir activity scores
- Fibrosis scores remained relatively stable over time in the small number of patients studied
Serious Adverse Events
- Only 2 of 71 serious adverse events (SAEs) were assessed as possibly related to interferon-alfa-2b/ribavirin treatment (Table 4)

|
| |
|
 |
 |
|
|