| |
Hepatotoxicity of antiretrovirals: Incidence, mechanisms and management
|
| |
| |
Journal of Hepatology
Volume 44, Issue (Supplement 1), Pages S132-S139 (2006)
Marina Nunez
Infectious Diseases, Instituto de Salud Carlos III, Madrid, Spain
One of the toxicities linked to the use of antiretrovirals is the elevation of transaminases. Liver toxicity is a cause of morbidity, mortality, and treatment discontinuation in HIV-infected patients. While several antiretrovirals have been reported to cause fatal acute hepatitis, they most often cause asymptomatic elevations of transaminases. Liver toxicity is more frequent among subjects with chronic hepatitis C and/or B. The incidence of drug-induced liver toxicity is not well known for most antiretrovirals. The contribution of each particular drug to the development of hepatotoxicity in a HAART regimen is difficult to determine. Possible pathogenic mechanisms involved in hepatotoxicity are multiple, including direct drug toxicity, immune reconstitution in the presence of HCV and/or HBV co-infections, hypersensitivity reactions with liver involvement, and mitochondrial toxicity. Other pathogenic pathways may be involved, such as insulin resistance caused by several antiretrovirals, which may contribute to the development of steatohepatitis. The management of liver toxicity is based mainly on its clinical impact, severity and pathogenic mechanism.
Article Outline
- Abstract
- 1. Introduction
- 2. Clinical impact
- 3. Definition of liver injury
- 4. Incidence and risk factors
- 4.1. Hepatitis B and C co-infections
- 4.2. Antiretrovirals
- 4.2.1. Protease inhibitors
- 4.2.2. Nucleoside analogues reverse transcriptase inhibitors (NRTI)
- 4.2.3. Non-nucleoside analogues reverse transcriptase inhibitors
- 4.2.4. Other factors
- 5. Mechanisms of liver toxicity
- 5.1. Direct toxicity
- 5.2. Hypersensitivity reactions
- 5.3. Mitochondrial toxicity
- 5.4. Metabolic abnormalities
- 5.5. Immune reconstitution in HCV and/or HBV-infected patients
- 6. Therapeutic management
1. Introduction
Highly active antiretroviral therapy (HAART) has dramatically changed the course of HIV infection, having decreased the morbidity and mortality derived from classical opportunistic infections. As a counterweight to this positive impact, antiretroviral therapy (ART) carries along undesirable effects, which challenge the management of HIV-infected patients to a great extent. Among these, liver toxicity deserves a special attention since it often leads to HAART discontinuation; particularly in hepatitis C (HCV) and/or hepatitis B (HBV) co-infected patients. The mechanisms involved in HAART-derived liver toxicity are not well understood, which makes its management more difficult.
In this review, the incidence and clinical impact of antiretroviral liver toxicity are evaluated. Furthermore, the mechanisms involved are explored, with the aim of helping with the management of this complication.
2. Clinical impact
With the widespread use of HAART and the availability of more drugs, some of them perhaps more hepatotoxic, HAART-linked hepatotoxicity has been made evident over the past few years. Liver toxicity generates medical visits, work-up exams, and frequent hospital admissions, all of which increase expenses. In addition, hepatotoxicity hampers the maintenance of HIV suppression over time.
In a recent American study, which evaluated the causes of death of HIV-infected individuals, discontinuation of ART due to hepatotoxicity increased from 6% in 1996 to 31.8% in 1998-1999 among those mortalities [1]. More recently, Kramer and colleagues have highlighted the increase in the number of cases of fulminant liver failure in HIV/HCV-coinfected individuals during the HAART era, even after excluding patients with advanced liver disease and adjusting by alcohol intake [2].
The severity of liver toxicity ranges from the absence of symptoms to liver decompensation; and the outcome, from spontaneous resolution to liver failure and death [3]. Although in a study severe hepatotoxicity with acute hepatic necrosis was present in 2% of HIV+ patients dying due to hepatitis or other liver diseases, mainly among those with prior liver disease, most cases of liver toxicity is mild-to-moderate and asymptomatic [4].
Drug liver toxicity has impacted on the recommendations for antiretroviral therapy in certain scenarios. Thus, the use of nevirapine (NVP) has been recommended to be avoided as part of post-exposure prophylaxis regimens. The reason for that was the occurrence of fulminant hepatitis in two cases and severe liver toxicity in 12 other healthy subjects who received a NVP-including HAART regimen after HIV exposure [5,6]. However, NVP seems to be safe when administered to mother and child as a single dose for prevention of mother-to-child HIV transmission [7].
3. Definition of liver injury
The clinician thinks of liver damage when abnormalities in the liver tests are seen. There is a broad variability among studies in the criteria to categorize the severity of hepatotoxicity. We propose as the most accepted one, the AIDS Clinical Trials Group scale of liver toxicity [8]. According to it, patients with transaminases within normal limits at baseline are considered to develop hepatotoxicity when ALT and/or AST rise above the upper limits of normal (ULN). Severe hepatic injury (the primary study outcome) is defined as grade 3 or 4 change in AST and/or ALT levels during antiretroviral treatment. If AST and ALT grades were discordant, the highest should be used for classification purposes.
Many drugs increase Á-glutamiltranspeptidase (GGT) levels. This is often misinterpreted as a marker of liver damage, but the isolated elevation of this enzyme actually reflects enzyme induction. Only when associated with a proportional increase in alkaline phosphatase levels should it be considered as a cholestatic lesion. Bilirubin should not be considered itself as indicator of liver toxicicity it can be elevated due to a variety of reasons, such as hemolysis, fasting and certain drugs (e.g. indinavir and atazanavir) [9]. In addition to HAART-derived hepatotoxicity, some other conditions or drugs used in HIV infection, can cause elevations in the levels of liver enzymes and should be ruled out.
4. Incidence and risk factors
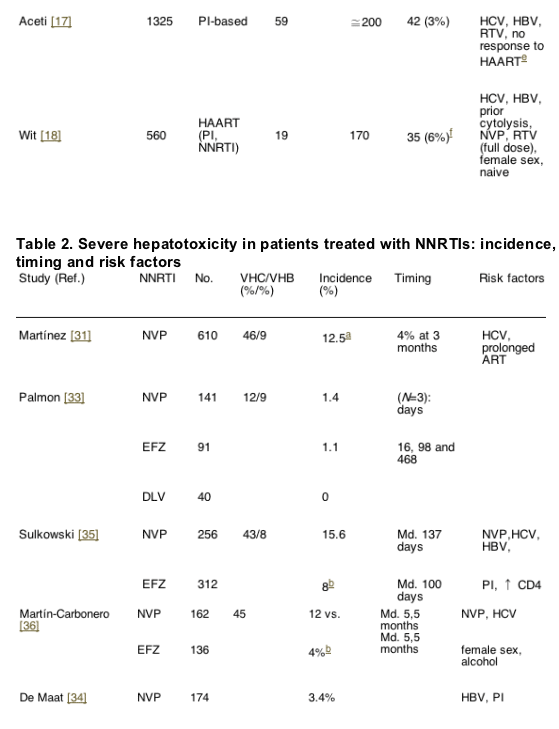
The reported incidence of severe liver toxicity after initiating HAART ranges from 2 to 18% [10-19]. Differences in the study populations, as well as in the methods used probably account for the wide range. In Tables 1 and 2, which summarize the main trials assessing liver toxicity in patients taking antiretroviral therapy, the risk factors are recorded.
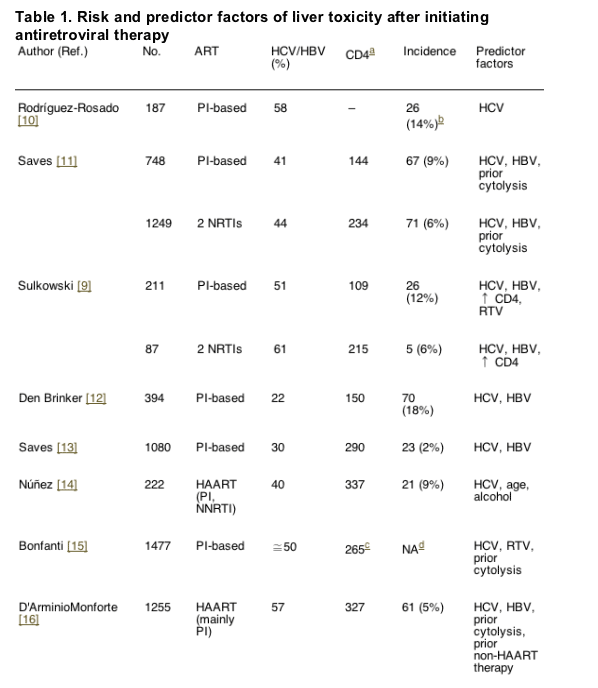
4.1. Hepatitis B and C co-infections
Liver toxicity, especially severe toxicity (grades 3 and 4), is clearly more frequent in HCV and/or HBV co-infected individuals treated with HAART [10-19]. In one study, a higher risk of hepatotoxicity was found in patients carrying HCV genotype 3 (HCV-3) compared to other genotypes [20]. More recently, other authors have obtained similar findings [21]. The clinical implications of this finding are 2-fold. On one hand, the presence of HCV-3 may impact on the selection of HAART regimen, choosing those with less potential for hepatotoxicity. On the other hand, since genotype 3 shows a higher response to ż-interferon (IFN) and ribavirin (RBV), anti-HCV treatment should be given if no major contraindication is present.
4.2. Antiretrovirals
The results of the studies that have evaluated the risk for liver toxicity associated with the use of particular antiretroviral drugs or families are conflicting. The unbalanced and often insufficient representation of some antiretrovirals in these series, make it difficult to determine with accuracy the role of each particular drug in the development of liver toxicity. In addition, the use of several antiretrovirals combined makes it difficult to ascribe the elevation of transaminases to single drugs.
4.2.1. Protease inhibitors
The phenomenon of hepatotoxicity became more evident after the introduction of ART of high activity, which initially included invariably a protease inhibitor (PI). However, none of the studies has been able to prove the higher potential for liver toxicity of this particular family of drugs. Among the PI, in some studies full-dose ritonavir (RTV) has been found to be more hepatotoxic [10,16,18,19,22], although these results have not been confirmed by others [17,23]. In certain cases, RTV has caused fatal acute hepatitis [24]. Several cases of liver toxicity associated with the use of indinavir (IDV) and saquinavir (SQV) have also been reported [25,26]. Nelfinavir was found to be less hepatotoxic than the other PI anlyzed (RTV, IDV, SQV y amprenavir (APV)) in study evaluating 1052 patients [27].
The use of two PI, which often includes RTV at low doses as a booster for the second PI, does not seem to increase the risk of toxicity for the liver [28,29]. The incidence of liver toxicity with lopinavir (LPV), which is given with low doses of RTV (200mg/day) is low [26,30,31]. Atazanavir, marketed more recently, seems also to have a good safety profile regarding the liver, even if used with low-dose RTV [32,33]. Tipranavir, recently approved, appears to be more hepatotoxic, most probably because it is given with higher doses of RTV (400mg/day) [34].
Note from Jules Levin: a study conducted by Mark Sulkowski found no difference between nelfinavir and Kaletra regarding the risk for severe hepatoxicity.
4.2.2. Nucleoside analogues reverse transcriptase inhibitors (NRTI)
Some authors have found a lower incidence of hepatotoxicity with lamivudine (3TC) and tenofovir [15,17]. However, the majority of the NRTI can induce mitochondrial damage, and, therefore, have a potential for the development of liver injury, as it will be explained below [35]. Cases of hepatic failure have been reported in patients taking zidovudine, but didanosine and stavudine have been most often involved in severe hepatotoxicity [36-39]. Abacavir (ABC) and tenofovir (TDF), with low potential for mitochondrial damage, seem to have a safer profile regarding the liver. In patients with chronic hepatitis B, the removal of 3TC may be accompanied by a flare of HBV replication, translated into an increase in transaminases.
4.2.3. Non-nucleoside analogues reverse transcriptase inhibitors
The risk of liver toxicity associated with the non-nucleoside analogues reverse transcriptase inhibitors (NNRTI) is variable and involves several aspects and mechanisms. Several cases of severe liver toxicity, some of them fatal, in subjects receiving NVP as part of a post-exposure prophylaxis regimen [5,6]. Likewise, in a trial assessing the NRTI emtricitabine (FTC), a higher incidence of hepatotoxicity was observed among patients taking NVP [40]. Of interest, in both series, the post-exposure prophylaxis and the FTC trial, hepatotoxicity developed early into treatment, and predominated among black women in the FTC study. These data suggest a hypersensitivity reaction causing the liver abnormalities. However, in other reports, the hepatotoxicity of NVP-containing regimens had a later onset (beyond the 4th month), with an increase in the cumulative incidence over time [41,42]. Therefore, it looks like there is a second mechanism through which NVP causes liver toxicity, much more common than the hypersensitivity syndrome.
Several retrospective studies have evaluated the development of hepatotoxicity linked to the use of NNRTI (Table 2). In some series, the incidence of liver toxicity is not higher compared to other antiretrovirals [41,43,44]. This is especially true in populations with a low prevalence of chronic HCV infection [43]. While some authors have found a higher risk of liver toxicity for NVP compared to efavirenz (EFZ) [45,46], others have failed to do so [43]. More recently, in a randomized clinical trial comparing NVP twice a day, NVP once a day and EFZ, higher incidences of severe hepatotoxicity were seen in the NVP groups (13.8% once a day and 7.2% twice a day) compared to the EFZ arm [47]. However, only the differences between the once a day NVP arm and the EFZ arm were statistically significant.
It is interesting that one of the studies did not find cross-hepatotoxicity between NVP and EFZ [45]. In the same study, the morbidity and mortality derived from liver toxicity among patients taking NVP or EFZ was similar. Moreover, in a study assessing NVP hepatotoxicity, transaminases decreased in many of the patients who continued taking the same treatment [41].
Taken together, all these data suggest that NNRTI have a greater risk to induce immunoallergic reactions involving the liver soon after initiation of therapy. With prolonged therapy, especially in HBV and/or HCV co-infected subjects, NNRTI have a trend to cause a slight increase in the cumulative incidence of hepatotoxicity, which may spontaneously abate over time. Only in rare occasions is liver toxicity serious. In particular, morbidity and mortality linked to the use of NVP has not been proven to be superior to those of other antiretrovirals.
4.2.4. Other factors
Heavy alcohol intake has also been identified as a risk factor for severe hepatotoxicity in patients taking antiretrovirals [15,46]. Several authors have identified other risk factors for the development of HAART-derived liver toxicity, such as the prior presence of transaminase elevation [12,16,17,19], older age [15], female sex [18,46], prior monotherapy [17], first antiretroviral treatment [19], lack of response to HAART (only observed at 12 months) [18], and an increase in the CD4 count after HAART initiation [10,45]. An association between advanced degrees of liver fibrosis and liver toxicity secondary to NNRTI has been recently reported [48].
5. Mechanisms of liver toxicity
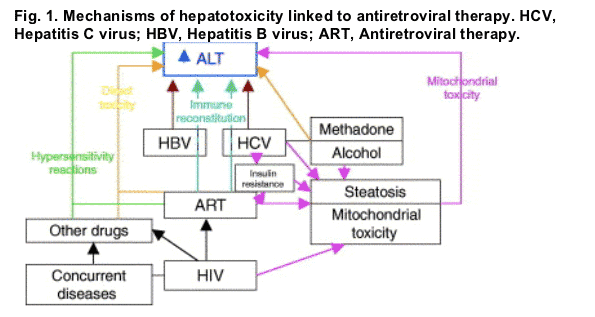
Despite the numerous published studies on antiretrovirals and hepatotoxicity, many unanswered questions still remain, in particular those related to the mechanisms involved. The possible mechanisms involved in the development of hepatotoxicity associated with the use of antiretrovirals are summarized in Fig. 1. It is probable that multiple pathogenic pathways simultaneously concur in some patients, being difficult to identify the exact mechanisms involved in the development of hepatotoxicity.
5.1. Direct toxicity
Antiretrovirals, as any other drug, can induce direct toxicity in the liver. Drugs metabolized in the liver through the cytocrom pathways may cause liver toxicity when there are polymorphisms in the enzymes [49]. Since many of the antiretrovirals are metabolized in the liver through the cytocrome pathways, idiosyncratic polymorphisms of the enzymatic complexes might lead to significant heterogeneity in drug metabolism, predisposing to the development of hepatotoxicity in certain individuals. Some drugs may potentiate the activation of death receptors and/or intracellular stress pathways [50]. Hepatocytes promote mechanisms of cytoprotection against the oxidative stress caused by drug metabolism. Heat-shock proteins, induced by various forms of stress including drugs, may exert cytoprotective functions helping to tolerate potentially damaging toxicants [49]. An increase in heat-shock proteins in individuals with polymorphisms may help the liver adapt to and minimize drug toxicity. Anti-oxidation stress mechanisms might explain the spontaneous normalization in the levels of transaminases despite maintenance of HAART, as it occurs with INH. Although still early in its development, pharmacogenomics is a new approach, which may be very valuable to predict the risk for hepatotoxicity in each individual after initiation of ART [51].
5.2. Hypersensitivity reactions
Hypersensitivity reactions are idiosyncratic reactions of the host, not related to the dose of the drug. Sulphas are prototypical drugs inducing these immune-mediated reactions involving the liver. They usually become apparent within the first 4-6 weeks of treatment. In patients taking NVP, the incidence of symptomatic events involving the liver has been reported to be of 4.9% [52]. The company marketing NVP has released a warning on the risk of severe toxicity, in some occasions with fatal outcome, linked to the use of NVP in women with CD4 counts >250cells/mm within 6 weeks after initiation of treatment, due to hypersensitivity reactions. According to a recent analysis, a low body mass index is an independent risk factor for this type of events [53].
Immune mediated drug reactions seem to involve the generation of neoantigens formed by the reaction of liver proteins with reactive drug metabolites [49]. Assays examining in vitro activation of peripheral blood mononuclear cells against a drug or its metabolites are currently under research. These assays are a promising approach to identify susceptible individuals [54]. On the other hand, individuals with the HLA-DRB1*0101 marker, especially if they have <25% CD4 cells, have been shown to be at greater risk of developing NVP-induced hypersensitivity reactions [55].
Hypersensitivity reactions have been reported relatively often with NVP and abacavir (ABC), both in HIV-infected patients and in subjects receiving prophylaxis after HIV-exposure [56-60], but also with other antiretrovirals such as zalcitabine (ddC) [61].
5.3. Mitochondrial toxicity
It is infrequent but a distinctive type of hepatoxicity that may evolve to acute liver failure. Mitochondrial toxicity is explained in another article. The main feature of the hepatic lesion is the accumulation of microvesicular steatosis in liver cells and mitochondrial depletion. This early lesion may evolve to macrovesicular steatosis with focal necrosis, fibrosis, cholestasis, proliferation of biliary ducts, and Mallory bodies, a clinical picture resembling alcohol-induced liver toxicity, pregnancy steatosis or Reye's syndrome [49]. Of interest, the underlying liver disease does not predispose to this type of lesion [49]. The ability of NRTI to inhibit mitochondrial DNA synthesis in vitro is in the following order: ddC, ddI, d4T, AZT, 3TC=ABC=TDF [62]. Hydroxyurea, used as coadjuvant treatment with ddI to enhance its activity, seems to increase the toxic effect of some NRTI, due to the rise of intracellular levels of 5tryphosphates products [63,64]. It is believed that the cumulative exposure to NRTI is an important factor for the development of lactic acidosis, since it usually appears after prolonged treatment, usually years, and correlates with the number of concomitant NRTI. In vitro data support an additive or synergistic long-term mitochondrial toxicity with some NRTI combinations [65].
5.4. Metabolic abnormalities
Steatohepatitis may cause hypertransaminasemia. Insulin resistance is believed to be the metabolic hallmark of predisposition to non-alcoholic steatohepatitis (NASH). HAART may cause, in the context of the lipodystrophy syndrome, marked abnormalities in the metabolism of both lipids and glucose, including insulin resistance [66]. Mild-to-moderate degrees of steatosis have been found in the liver of patients experiencing HAART-derived hepatotoxicity [67]. Thus, in some patients receiving HAART, insulin resistance and NASH may contribute to the development of liver toxicity.
Note from Jules Levin: studies in HIV-negative individuals find rosiglitazone and pioglitazone can reduce risks associated with metabolic syndrome and can reduce risks for cardiovascular disease associated with insulin resistance and diabetes, as well as in non-diabetics. Studies of atazanavir sjow it is associated with less risk than other PIs for metabolic syndrome, elevated lipids. As well, preclinical studies suggest that atazanavir may reduce risk for glucose anmormalities in HIV+ patients who have not yet developed glucose abnormalities compared to other PIs associated with risk for glucose abnormalities.
5.5. Immune reconstitution in HCV and/or HBV-infected patients
Liver damage induced by chronic HCV and HBV infection is mainly immune-mediated. The immune deficit caused by HIV infection is responsible for the attenuation of the inflammatory reaction in the liver of co-infected subjects. The inhibition of HIV replication with HAART leads to immune reconstitution, and consequently the immune response to HCV and/or HBV antigens exposed in the liver cell is also restored. Thus, HAART therapy may induce the development of hypertransaminasemia and even symptomatic hepatitis in patients with HCV [11,68-72] or HBV co-infection [73,74].
The immune reconstitution syndrome as a mechanism of liver toxicity is controversial, but there are several data supporting it. Markers of HCV-specific immune responses (HCV core-specific IGG antibody), T-cell activation and inflammation, have been found to correlate with liver damage and immune reconstitution [75]. Some authors have identified an increase in >50 CD4 cells per mm3 after initiating HAART as an independent risk factor for hepatotoxicity [10,45]. Liver histology has shown exacerbated viral hepatitis in some patients developing severe hypertransaminasemia while on HAART [67]
6. Therapeutic management
The three main considerations necessary for the management of transaminase elevation after the introduction of HAART are severity, clinical impact and etiologic mechanisms. Suspicion of hypersensitivity reactions or lactic acidosis, and the presence of liver decompensation, are all reasons to stop treatment [76]. Severe liver toxicity (grades 3-4), even in the absence of symptoms warrants discontinuation of the antiretroviral therapy.
In the remaining cases, decisions should be made on an individual basis. If liver steatosis is present, it is indicated to pursue the removal of predisposing factors. If the patient is taking an antiretroviral with higher hepatotoxic potential, substitution of that particular drug is an available option. Regarding the continuation of the same regimen, spontaneous decrease of transaminases levels has been reported [77]. However, the elevation of transaminases can persist, and the long-term consequences are unknown. In that regard, the finding of an association between NVP use and hepatic fibrosis is worrisome [78]. Nevertheless, this is a single study, and its results should be confirmed by other investigations.
|
|
| |
| |
|
|
|