| |
Entecavir 96 weeks in HBeAg(-), study ETV-027
|
| |
| |
Reported by Jules Levin
Poster at Shanghai - Hong Kong International Liver Congress 2006
This data was previously presented at APASL, Manila, Philippines, 2006
Ching-Lung Lai1, Ting-Tsung Chang2, You-Chen Chao3, Tawesak Tanwandee4, Satawat Thongsawat5, Shou-Dong Lee6, Peter Angus7, Richard Zink8, Jin Zhu8, Helena Brett-Smith8, Boon-Leong Neo9 and the BEHoLD Study Group
1Queen Mary Hospital, Hong Kong, China 2National Cheng Kung University Medical College, Tainan, Taiwan 3Tri-Service General Hospital, Taipei, Taiwan 4Siriraj Hospital, Mahidol University, Bangkok, Thailand
5Maharajnakorn Chiang Mai Hospital, Chiang Mai, Thailand 6Taipei Veterans General Hospital, Taipei, Taiwan 7Austin & Repatriation Medical Centre, West Heidelberg, VIC, Australia 8Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, CT, USA 9Bristol-Myers Squibb Pharmaceutical Research Institute, Singapore
Background
Entecavir (ETV) is a potent and selective inhibitor of hepatitis B virus (HBV) polymerase which inhibits priming, reverse transcription, and DNA synthesis1
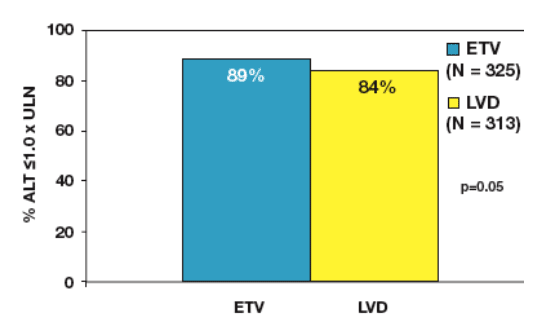
In this Phase III trial (ETV-027), ETV demonstrated superior virologic, histologic and biochemical benefit compared to lamivudine (LVD) after 48 weeks of therapy in nucleoside-naive HBeAg(-) patients2 (Table 1)
ETV was well tolerated and the safety profile was comparable to that of LVD2
Efficacy and safety through Week-96 are presented here
Methods
Study Design
- Patients were randomized (1:1) to receive either ETV 0.5 mg (N = 325) or LVD 100 mg (N = 313) for 48 weeks
- At week 52, patient management decisions were made based on HBV DNA by bDNA and ALT obtained at week 48:
- Responders (HBV DNA by bDNA <0.7 MEq/mL and ALT <1.25 x ULN) discontinued therapy and were followed 24 weeks off-treatment
- Virologic responders (HBV DNAby bDNA <0.7 MEq/mL and ≥1.25 x ULN) continued into year 2 and received blinded treatment up to week 96
- Non-responders (HBV DNAby bDNA ≥0.7 MEq/mL) discontinued therapy
- During year 2 of dosing (Week 52-96), virologic responders who became
responders or non-responders (with 2 sequential measurements of HBV DNAby
bDNA≥0.7 MEq/mL) discontinued therapy at the time response was identified
Study Endpoints Through Week 96
Study endpoints through Week 96 included:
- HBV DNA <300 copies/mL by PCR
- ALT normalization (≦1.0 x ULN)
- Safety
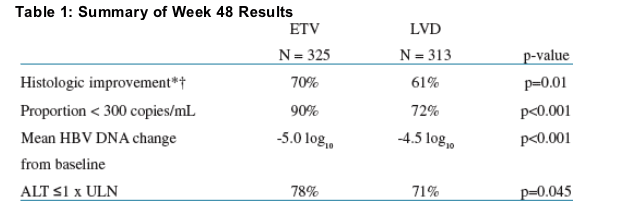
* Histologic Improvement: ≥2-point improvement in Knodell Necroinflammatory score with no worsening in Knodell Fibrosis score.
ETV N = 296; LVD N = 287 due to number of evaluable biopsies.
Results: Year 2 Treatment Cohort
- The year 2 treatment cohort constituted the virologic responders at Week 48,
(ETV = 26; and LVD = 28) who continued to a second year of therapy
Proportion of patients with HBV DNA <300 copies/mL at Week 48 and
through Week 96
- 25 (96%) of ETV-treated patients who continued into the second year of
maintained HBV DNA <300 copies/mL by the end of dosing (Figure 2)
- At both Week 48 and end of dosing, 18 (64%) of LVD-treated patients
achieved HBV DNA <300 copies/mL
Figure 2: Year 2 Treatment Cohort: Proportion with HBV DNA <300 copies/mL at Week 48 and Through Week 96
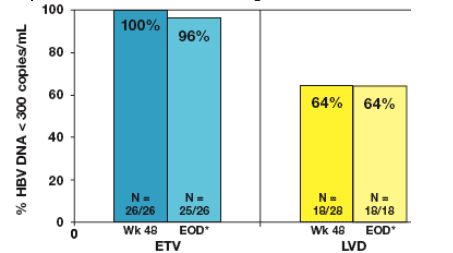
*EOD (end of dosing) is defined as the last observation on-treatment
Proportion of Patients with ALT ≦1 x ULN at Week 48 and Through Week 96
27% of ETV-treated patients and 21% of LVD-treated patients achieved
ALT normalization during the second year of treatment (Figure 3)
Figure 3: Year 2 Treatment Cohort: ALT ≦1 x ULN at Week 48 and Through Week 96
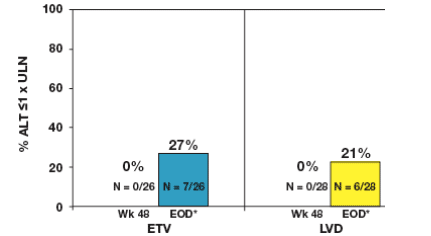
*EOD (end of dosing) is defined as the last observation on-treatment
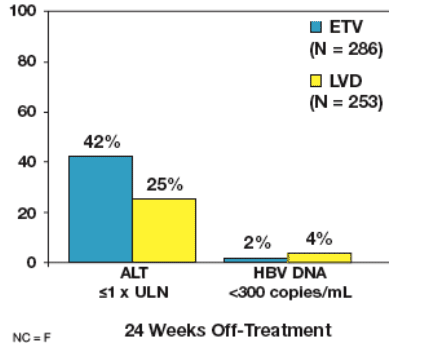
Off-Treatment Results: Responders
Response 24 weeks off-treatment was evaluated among patients achieving
a Response (HBV DNA <0.7 MEq/mL by bDNA and ALT <1.25 x ULN)
at 48 weeks (ETV N = 275; LVD N = 245) and through 96 weeks (ETV
N = 11; LVD N = 8)
Not all responders achieved these endpoints at the time of treatment
discontinuation
Figure 4: Responders: 24 Weeks Off-Treatment
Patients who Entered ETV-901 (roll-over)
- Patients who experienced recurrence of viremia by bDNA during the offtreatment follow-up period in ETV-027 were eligible to enter into ETV-901
- ETV 901 is a roll-over study providing ETV plus LVD (subsequently changed to ETV monotherapy) in phase III patients who met criteria for continued or resumed treatment
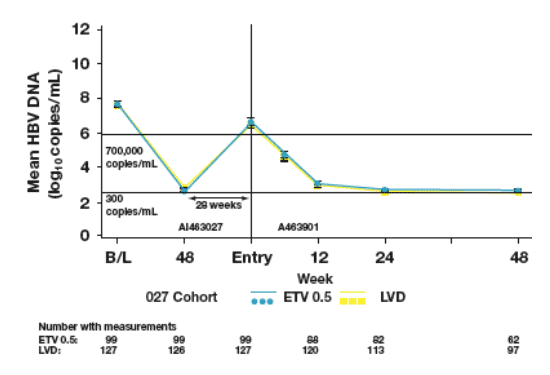
Mean HBV DNA
- Patients who entered ETV-901 demonstrated an early reduction in mean
HBV DNA levels by Week 12, and maintained HBV DNA suppression through Week 48
Figure 5: Patients who Entered ETV-901 from Off-treatment Period: Mean HBV DNA
HBV DNA <300 copies/mL
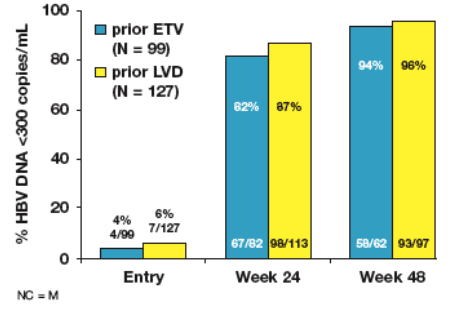
At Week 48, over 90% of patients who restarted ETV treatment achieved HBV
DNA<300 copies/mL compared to under 10% at study entry (Figure 6)
Figure 6: Patients who Entered ETV-901 from Off-treatment Period:
HBV DNA <300 copies/mL
Results: All Treated Patients
- In order to assess the cumulative probability of response among naive
HBeAg(-) patients who start treatment with ETV versus LVD, cumulative confirmed response rates over 2 years of treatment are provided for the
all-treated cohort (ETV N = 325, LVD N = 313)
- Cumulative confirmed response was defined as:
- Cumulative proportion of treated subjects who ever achieved a confirmed endpoint on treatment
- Confirmed = 2 sequential measurements which meet success criteria (or last observation)
Figure 7: All Treated Patients: Cumulative Confirmed HBV DNA <300 copies/mL Through 96 Weeks
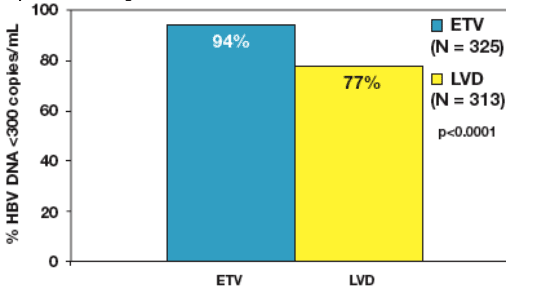
Figure 8: All Treated Patients: Cumulative Confirmed ALT ≦1 x ULN Through 96 Weeks
Safety
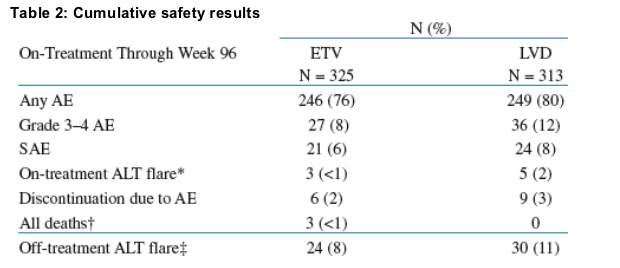
- Overall the on-treatment safety profiles were comparable between ETV and LVD (Table 2)
- Fewer discontinuations due to AEs were observed for ETV-treated patients (Table 2)
- The incidence of ALT flares was lower in the ETV-treated patients, both
on- and off-treatment (Table 2)
- Overall, ETV was well tolerated through 96 weeks
Table 2: Cumulative safety results
* On-treatment ALT flare = ALT >2 x BL and >10 x ULN
On-treatment and during follow-up. No death was attributed to study therapy
On-treatment ALT flare = ALT >2 x reference and >10 x ULN
Conclusions
- 85% of ETV-treated patients (compared to 79% LVD-treated patients) in
ETV-027 achieved response by Week 48 and discontinued dosing
- Extended dosing into the second year maintained virologic suppression and improved ALT normalization
- Despite profound virologic suppression during the first year of dosing, most patients experienced recurrence of viremia by bDNA during offtreatment
follow-up
- Patients with recurrent viremia off-treatment responded promptly to ETV
retreatment in ETV-901
- For all treated patients through 96 weeks, a higher cumulative proportion
of patients in the ETV (94%) group than in the LVD (77%) group achieved confirmed HBV DNA <300 copies/mL (p<0.0001)
- No ETV resistance was identified through 96 weeks of treatment in patients with no LVDr substitutions at baseline (please see oral presentation by Colonno R, et al. Shanghai-Hong Kong ILC 2006)
- Safety profiles were comparable between ETV-treated and LVD-treated patients through 96 weeks of treatment with low incidence of on- and
off-treatment flares
References
1. Seifer M, et al. Antimicrob Agents Chemother. 1998;42:3200-3208
2. Shouval D, et al. Hepatology 2004;40(Suppl 1):728A
|
|
| |
| |
|
|
|