| |
Entecavir vs Lamivudine in 515 Treatment-Naives: safety & efficacy
|
| |
| |
"Entecavir (ETV) Is Superior To Lamivudine (LVD) For The Treatment Of Chronic Hepatitis B
(CHB): Results Of A Phase 3 Chinese Study (ETV-023) In Nucleoside-Naive Patients"
Reported by Jules Levin
41st Annual Meeting of the European Association for the Study of the Liver
April 26-30, 2006, Vienna, Austria
Authors: Guangbi Yao1, ChengWei Chen2, WeiLun Lu3, Hong Ren4, DeMing Tan5, YuMing Wang6, DaoZheng Xu7, Michael Jiang8, Jessica Liu8, Dong Xu9, Laurie MacDonald9
1Shanghai Jing An Central Hospital, Shanghai, China; 2Shanghai Liver Disease Center Of Nangjing Military Area, Shanghai, China; 3No. 3 Hospital Affiliated to Zhongshan Medical University, Guangzhou, China; 4No. 2 Hospital Affiliated to Chongqing Medical University, Chongqing, China; 5Infectious
Disease Dept. of Xiang Ya Hospital, Changsha, China; 6Xinan Hospital, Chongqing, China; 7Beijing Di Tan Hospital, Beijing, China; 8Bristol-Myers Squibb Company, Shanghai, China; 9Bristol-Myers Squibb Company, Wallingford, CT, USA.
ENTECAVIR RESULTS IN EARLY VIRAL LOAD REDUCTION IN CHRONIC HEPATITIS B PATIENTS WHO HAVE FAILED LAMIVUDINE THERAPY: A RANDOMIZED, PLACEBO-CONTROLLED STUDY IN CHINA
G. Yao1, X. Zhou2, D. Xu3, B. Wang4, H. Ren5, M. Jiang6, J. Liu6, D. Xu7, L. MacDonald7
1Shanghai Jing An Central Hospital, Shanghai 2Rui Jin Hospital Affiliated to Shanghai No.2 Medical University, Shanghai 3Beijing Di Tan Hospital, Beijing 4Beijing Friendship Hospital Affiliated to Capital Medical University, Beijing 5No. 2 Hospital Affiliated to Chongqing Medical University, Chongqing 6Bristol-Myers Squibb Company, Shanghai, China 7Bristol-Myers Squibb Company, Wallingford, CT, USA
Background: Entecavir (ETV) is a potent inhibitor of both wild-type and lamivudine (LVD)-resistant hepatitis B virus (HBV) with proven clinical efficacy. We conducted a randomized, double-blind, multi-center study in China (ETV-056) in adult patients with chronic hepatitis B infection (CHB) who were refractory to LVD therapy.
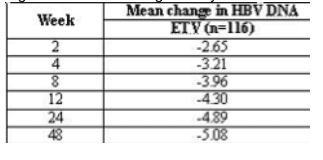
Methods: 145 patients with CHB who had failed prior LVD therapy were treated with ETV 1.0 mg (116 patients) or placebo (29 patients) for 12 weeks in the double-blind dosing phase of the study. All patients then received open-label ETV for 36 weeks. The primary endpoint was the mean change in HBV DNA from baseline at Week 12. HBV DNA levels measured by Roche Amplicor■ HBV DNA PCR assay were obtained at weeks 2, 4, 8 and 12 in the double blind phase and weeks 24 and 48 of the open-label phase.
Results: The mean change in HBV DNA from baseline at week 12 was -4.30 log10 copies/mL for ETV and -0.15 log10 copies/mL for PBO (p < 0.0001). Treatment with ETV 1.0 mg produced rapid reductions in HBV DNA.
After 48 weeks of ETV treatment, 27% of patients had undetectable HBV DNA levels (< 300 copies/mL). Among patients with elevated ALT at baseline, 85% had achieved normalization of ALT after 48 weeks of ETV treatment. ETV 1.0 mg for 48 weeks was generally well tolerated.
Conclusions: ETV is a potent antiviral agent that achieves rapid and profound reductions in HBV DNA in patients who had failed prior treatment with LVD.
STUDY 023: Entecavir vs Lamivudine in Treatment-Naives in China
ABSTRACT
Background: Entecavir is a potent inhibitor of hepatitis B virus (HBV) with proven clinical efficacy. A randomized, double-blind, multi-center study was conducted in China in nucleoside-naive patients with CHB.
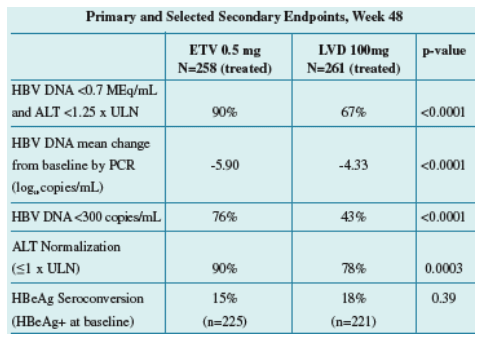
Methods: 519 patients were treated with ETV 0.5mg (n=258) or LVD 100mg (n=261) for at least 52 weeks. The primary endpoint was a composite
endpoint of HBV DNA <0.7 MEq/mL by bDNA assay and ALT <1.25 xULN at Week 48. HBV DNA-levels as measured by the Roche Amplicor■
PCR assay were obtained at weeks 12, 24, 36 and 48. 0.7 MEq/mL approx. equal to 700,000 copies/mL,
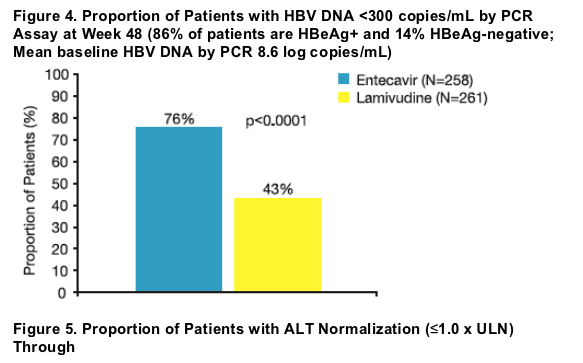
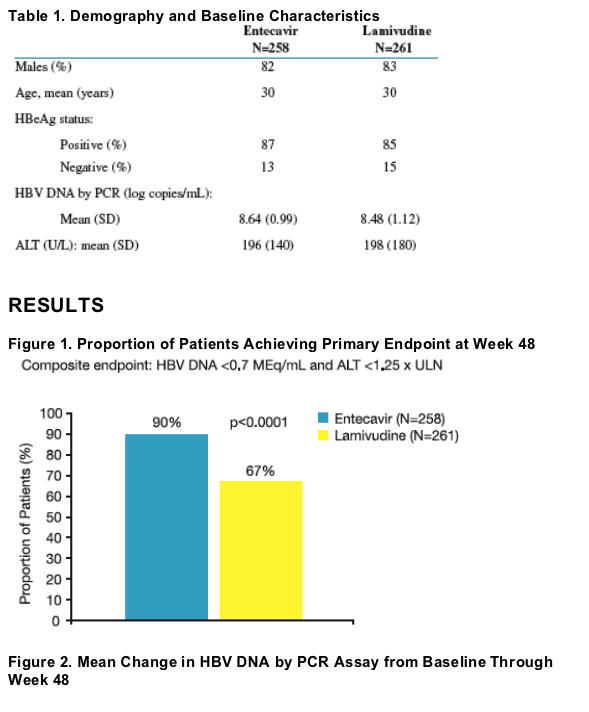
Results: Baseline characteristics were well balanced between treatment groups. Of treated patients, 86% were HBeAg-positive, mean HBV DNA by PCR was 8.56 log10 c/mL, and mean ALT was 197 U/L. Results for the primary and key
secondary endpoints are presented below:
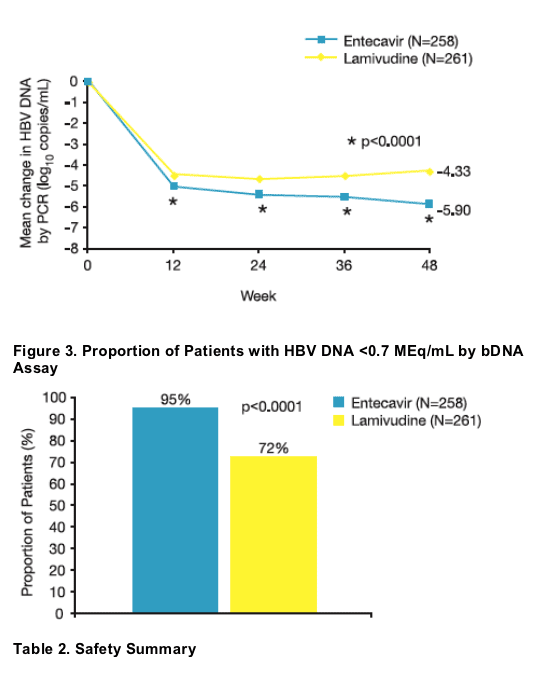
Treatment with ETV produced rapid reductions in HBV DNA levels. ETV was superior to LVD for the mean change in HBV DNA from baseline at every time point measured (p <0.0001).
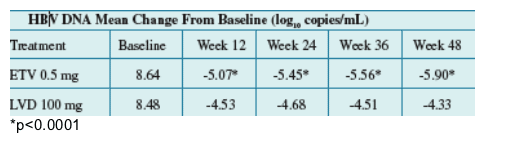
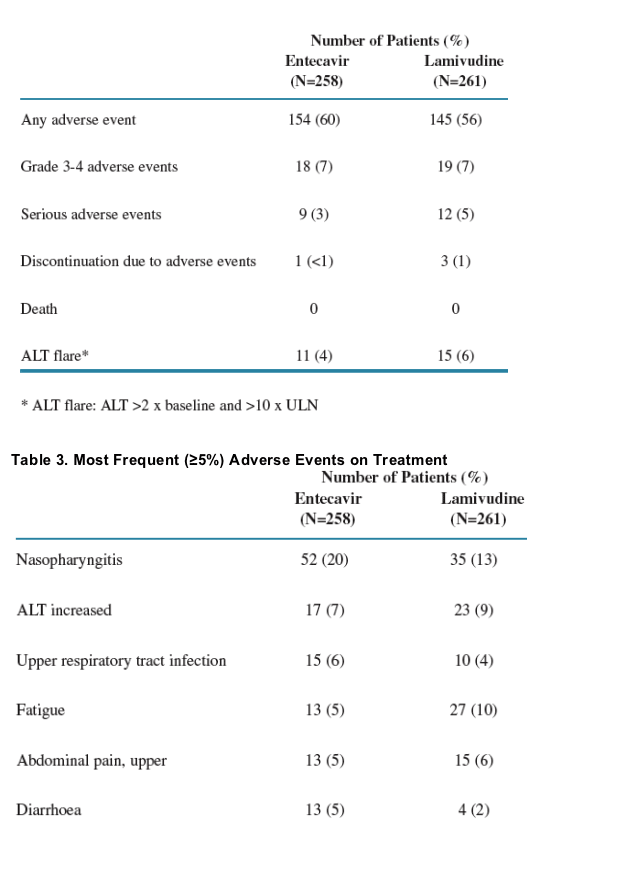
Overall incidence of adverse events (AEs) was comparable: 60% of ETV patients and 56% of LVD patients reported adverse events; 3% of ETV patients and 5% of LVD patients reported serious AEs.
Conclusions: ETV achieves superior virologic and biochemical improvements in nucleoside-naive patients with CHB with a safety profile comparable to LVD.
Study Objective
To compare the efficacy and safety of entecavir (0.5 mg QD) to that of lamivudine (100 mg QD) in nucleoside-naive, Chinese patients with CHB
Study Design
- Phase III, double-blind, randomized (1:1) study of entecavir 0.5 mg QD (N=258) vs. lamivudine 100 mg QD (N=261) for at least 52 weeks
- HBeAg-positive and HBeAg-negative patients were enrolled from 26 sites across China
- Primary efficacy analyses were based on results at Week 48
Key Inclusion Criteria
- Age ≥16 years
- HBsAg-positive for >24 weeks prior to screening
- HBeAg-positive or HBeAg-negative/HBeAbpositive
- HBV DNA >3 MEq/mL by bDNA
- ALT 1.3-10 x ULN
- Compensated liver disease
- Nucleoside treatment naive
Efficacy Endpoints
Primary Endpoint
- Composite endpoint: HBV DNA <0.7 MEq/mL by bDNA assay and ALT <1.25 x ULN at Week 48
Secondary Endpoints
- Mean change from baseline in HBV DNA by PCR assay (log10 copies/mL)
- HBV DNA <0.7 MEq/mL by bDNA assay
- HBV DNA <300 copies/mL by PCR assay
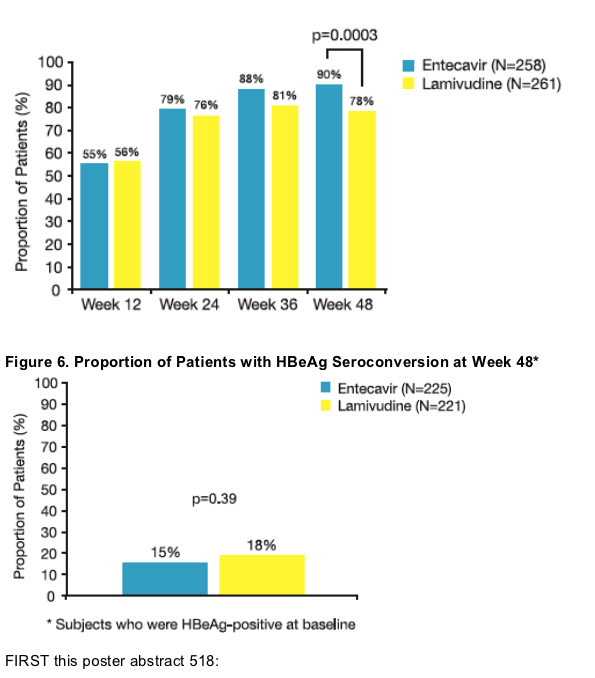
- HBeAg seroconversion
- ALT <1.0 x ULN
|
|
| |
| |
|
|
|