| |
HIV <500 May Be Less Risky than >500
|
| |
| |
Is Moderate HIV Viremia Associated With a Higher Risk of Clinical Progression in HIV-Infected People Treated With Highly Active Antiretroviral Therapy: Evidence From the Italian Cohort of Antiretroviral-Naive Patients Study
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 41(1) 1 January 2006 pp 23-30
Murri, Rita MD*; Lepri, Alessandro Cozzi PhD ; Cicconi, Paola MD ; Poggio, Antonio MD ; Arlotti, Massimo MD ; Tositti, Giulia MD ; Santoro, Domenico MD#; Soranzo, Maria Luisa MD**; Rizzardini, Giuliano MD ; Colangeli, Vincenzo MD ; Montroni, Maria MD ; Monforte, Antonella D'Arminio MD ; for the ICoNA Study Group
From the *Istituto di Clinica delle Malattie Infettive, Universita Cattolica S. Cuore of Rome, Rome, Italy; Royal Free Centre for HIV Medicine and Department of Primary Care and Population Sciences, Royal Free and University College Medical School, Royal Free Campus, London, United Kingdom; Istituto di Malattie Infettive e Tropicali, Universita di Milano, Milan, Italy; Divisione Malattie Infettive, Ospedale Civile di Verbania, Verbania, Italy; Divisione Malattie Infettive, Ospedale degli Infermi di Rimini, Rimini, Italy; Divisione Malattie Infettive, Ospedale di Vicenza, Vicenza, Italy; #Divisione Malattie Infettive, Ospedale Sant'Anna di Como, Como, Italy; **Divisione Malattie Infettive B, Ospedale Amedeo di Savoia di Torino, Torino, Italy; Divisione Malattie Infettive, Ospedale di Busto Arsizio, Arsizio, Italy; Istituto di Malattie Infettive, Universita di Bologna, Bologna, Italy; and Servizio Regionale di Immunologia, Ospedale Regionale Generale di Ancona, Ancona, Italy.
Here are selected quotes from the authors of this interesting study whose findings suggest that full suppression of HIV viral load is better in terms of clinical outcome than any detectable viral load:
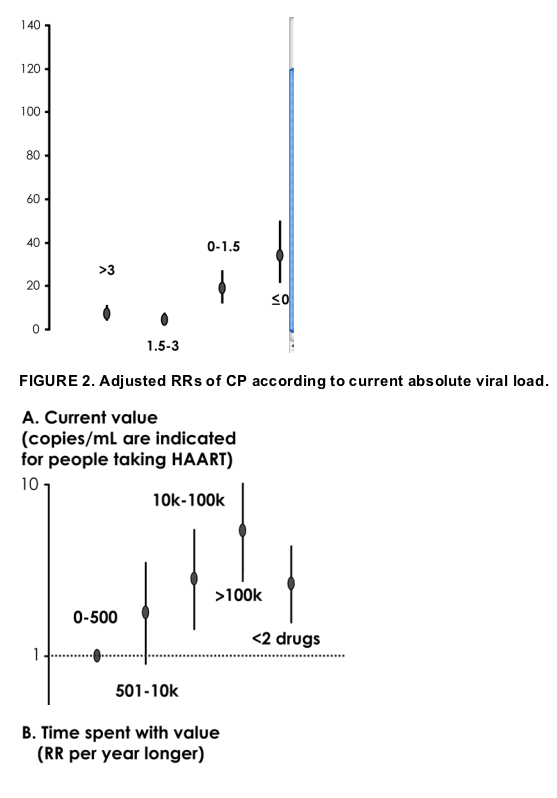
.....The risk of CP (clinical progression) was higher in patients with a current viral load ranging between 10,000 and 100,000 copies/mL (RR = 2.79, 95% CI: 1.43 to 5.44; P = 0.003) or >100,000 copies/mL (RR = 5.34, 95% CI: 2.83 to 10.08; P = 0.0001) compared with those with a current viral load ≦500 copies/mL (see Fig. 2A).
In contrast, compared with patients with a current suppressed viral load, the risk of CP seemed higher in patients with a viral load of 501 to 10,000 copies/mL, but the association was not statistically significant at the 0.05 level (RR = 1.77, 95% CI: 0.90 to 3.51; P = 0.09). When we fit the variable as an ordinal covariate, we found strong evidence for the existence of a linear trend (RR = 1.90 per unit increase in the ordinal variable, 95% CI: 1.54 to 2.35; P = 0.0001).....
.... it has been shown that therapy interruption is associated with a high immediate risk of CP, whereas continued therapy, especially with a PI, seems to be associated with protection against opportunistic infections over and above that conferred by the increase in CD4 count and decrease in viral load alone. Moreover, Deeks et al have shown that in patients with detectable viremia, an immunologic deterioration may occur but only after a prolonged period (3 years)......
... Current guidelines recommend a switch of therapy as soon as virologic failure is confirmed, and complete viral suppression remains the ultimate goal of therapy, especially in antiretroviral-naive people. If it is possible to construct a new regimen that is potent virologically (eg, contains ≥3 active drugs) and is expected to be easily accepted by the patient, this is certainly the recommended strategy. This is not always possible, however; some patients have limited options because they carry a multiresistant virus or are intolerant to specific drugs.....
Read the Results & Author Discussion below and view the graphs below for a full contextual review of these study results and clinical decision-making.
Abstract
Objective: To assess the risk of clinical progression (CP) according to the duration of time spent without complete viral load (VL) suppression compared with that associated with periods of stably suppressed viremia in HIV-infected people who started highly active antiretroviral therapy (HAART) when previously naive to antiretrovirals.
Design: A cohort study of patients having started HAART after enrollment in the Italian Cohort of Antiretroviral-Naive Patients (ICoNA) and being followed for at least 6 months.
Methods: Person-years spent in different categories according to the VL level and the change in VL from the most recent value before the initiation of HAART were calculated. A multivariable Poisson regression model, including potential confounders, was constructed.
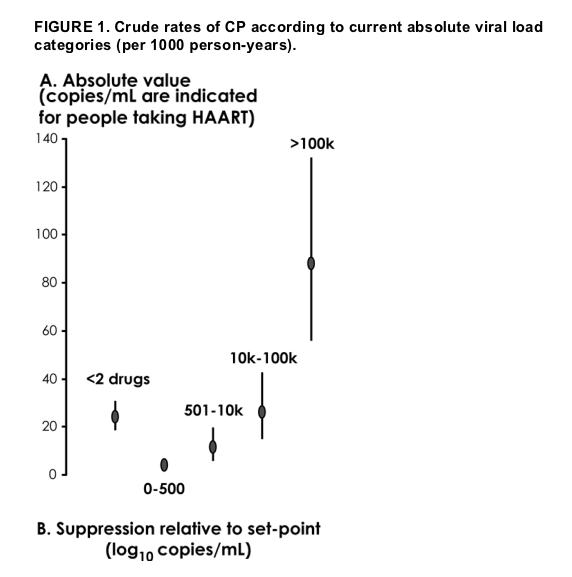
Results: A total of 3023 patients were studied. The overall rate of CP was 13.4 per 1000 person-years.
Evidence for a higher risk of CP was observed for people with a current VL >10,000 copies/mL. For each year longer spent on HAART with a VL >100,000 copies/mL, a 5-fold increased risk was observed (relative risk [RR] = 5.34, 95% confidence interval [CI]: 2.83 to 1.08; P = 0.0001).
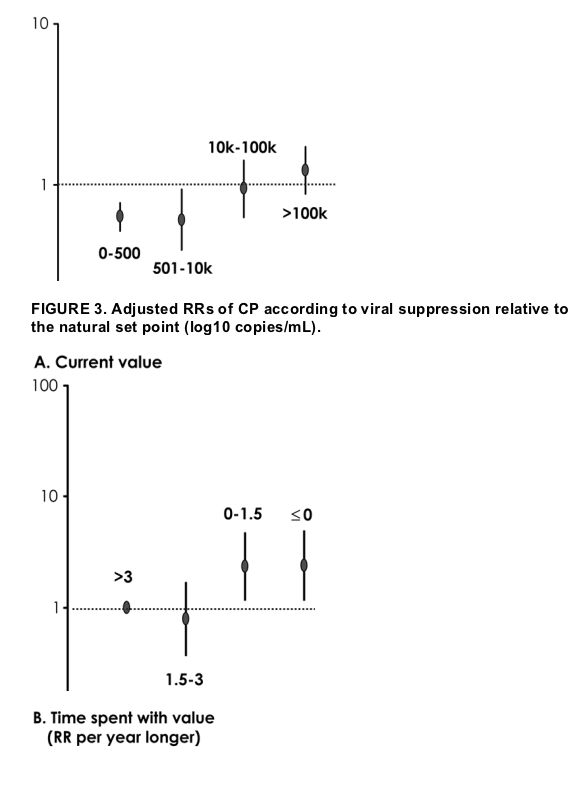
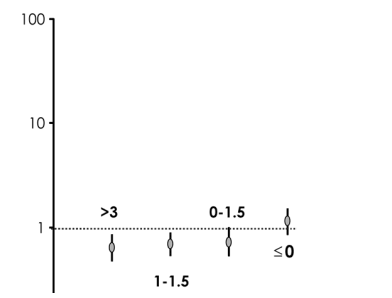
An increased risk of CP in patients with current suppression <1.5 log10 copies/mL (RR = 2.34, 95% CI: 1.16 to 4.74; P = 0.02) and in those with no suppression or a VL higher than their set point (RR = 2.39, 95% CI: 1.17 to 4.89; P = 0.02) was observed compared with those with suppression of >3 log10 copies/mL, although it was not significant. Longer duration on HAART with a VL suppressed below set point seemed to confer protection against CP.
Conclusions: Virologic failure to antiretroviral drugs is common. The risk of CP may remain low despite a low but detectable level of HIV viremia.
Rationale for Study
Achieving and maintaining viral suppression less than 50 copies/mL is the goal of current antiretroviral regimens.1 A significant proportion of patients eventually experience virologic failure, however.2 The reference point of 50 copies/mL reflects the lower limit of detection for the viral load assays currently available; however, the threshold at which therapy should be switched to optimize patients' future prognosis is unknown. In a recent study of treated HIV-infected patients with stable detectable viral replication (<10,000 copies/mL),3 the authors demonstrated that clinical and immunologic benefits were maintained in patients with partial virologic suppression, confirming similar results from previous published reports.4-7 In another study, Deeks et al8 suggested that the selection of drug resistance mutations in patients taking highly active antiretroviral therapy (HAART) with low to moderate levels of viremia might reduce HIV pathogenicity. Further, there is evidence that treatment with protease inhibitor (PI)-containing HAART confers protection against clinical progression (CP) over and above what can be explained by the CD4 count increase induced by therapy.9 A recent report showed that patients who maintained an HIV-1 RNA level of 400 to 20,000 copies/mL preserved immunologic status and were no more likely to die or develop a new AIDS-defining diagnosis than those with baseline levels <400 copies/mL.10 Patients were grouped according to a 6-month period randomly selected during the follow-up period, however, such that the whole story of ongoing viral suppression during follow-up was not properly accounted for.
Current treatment guidelines suggest that HAART should be changed after 2 consecutive HIV viral load measurements greater than 400 copies/mL if tolerable and effective treatment is available.1 The rationale for changing therapy at such a point in time is related to the attempt to reduce the chance of developing drug resistance to the failing regimen, and potentially to the following regimens. A switch in therapy poses new challenges for tolerability, adherence to a new regimen, and fit of therapy into the patient's daily routine, however. Indeed, despite published recommendations, it seems common in clinical practice that partial loss of viral suppression is tolerated without an immediate change in regimen, especially in patients who have limited therapy options.
Based on these previous observations, we planned an analysis of patients previously naive to antiretrovirals and enrolled in the Italian Cohort of Antiretroviral-Naive Patients (ICoNA) to study the risk of CP according to the duration of time spent without full viral load suppression as well as according to the degree of viral suppression from patients' pretherapy levels compared with that associated with periods of stably suppressed viremia.
RESULTS
A total of 3023 patients were studied. The follow-up was rigorous, with the percentage of the population not seen at any of the ICoNA centers for >1 year being 28.5%. The characteristics of the study population 6 months after starting HAART (time 0 of the analysis) are shown in Table 1. We had observed the patients for a cumulative time of 11,447 person-years of follow-up (PYFU). Of these, 2669 PYFU (23.3%) were spent off HAART (on single or dual ART or therapy interruptions), 6775 PYFU (59.2%) with a viral load <500 copies/mL, 1130 PYFU (9.9%) with a viral load between 501 and 10,000 copies/mL, 612 PYFU (5.4%) with a viral load between 10,001 and 100,000 copies/mL, and 261 PYFU (2.3%) with a viral load >100,000 copies/mL.
As to the general suppression relative to the viral set point at which patients were receiving HAART over the follow-up period, we observed a total of 710 PYFU (6.2%) with a viral load equal to or above the natural set point, 1437 PYFU (12.6%) with a maximum suppression of 1.51 log10 copies/mL below the set point, 3708 PYFU (32.4%) with a suppression ranging between 1.51 and 3 log10 copies/mL, and 2923 PYFU (25.5%) with suppression more than 3 log10 relative to the set point.
Over a median clinical follow-up of 46 months (range: 1-81 months), we registered 40 HIV-related deaths and 113 patients with a new AIDS-defining disease, for a total of 153 events. The AIDS-defining diseases and the reported cause of death for these 153 events are listed in Table 2. Therefore, the overall rate of CP was 153 events per 11,447 person-years (13.4 per 1000 person-years; 95% confidence interval [CI]: 11.3 to 15.7).
The crude rates of CP according to the current viral load categories as defined in the Patients and Methods section are shown in Figure 1A. Figure 1B shows the same figures; however, here, the rates were stratified according to the ongoing viral load suppression relative to patients' natural set point. The risk of CP was relatively low in patients with a current viral load <10,000 copies/mL (-12 per 1000 person-years) or a suppression >1.5 log10 below the natural set point (-6 per 1000 person-years).
Figures 2 and 3 show the adjusted RRs of CP from fitting the multivariable Poisson regression model, including all the other factors stated in the Patients and Methods section (age, gender, mode of HIV transmission, CD4 nadir before ART initiation, calendar year of HAART initiation, clinical stage at entry, previous history of hospitalization, use of opportunistic infection prophylaxis before ART initiation, current viral load, duration of time spent in each viral load stratum, cumulative number of drugs started during follow-up, current use of an NNRTI or PI, and current HCV serology). The risk of CP was higher in patients with a current viral load ranging between 10,000 and 100,000 copies/mL (RR = 2.79, 95% CI: 1.43 to 5.44; P = 0.003) or >100,000 copies/mL (RR = 5.34, 95% CI: 2.83 to 10.08; P = 0.0001) compared with those with a current viral load ≦500 copies/mL (see Fig. 2A). In contrast, compared with patients with a current suppressed viral load, the risk of CP seemed higher in patients with a viral load of 501 to 10,000 copies/mL, but the association was not statistically significant at the 0.05 level (RR = 1.77, 95% CI: 0.90 to 3.51; P = 0.09). When we fit the variable as an ordinal covariate, we found strong evidence for the existence of a linear trend (RR = 1.90 per unit increase in the ordinal variable, 95% CI: 1.54 to 2.35; P = 0.0001).
The covariates indicating the current duration of time spent with a viral load >10,000 copies/mL while patients were treated with HAART showed a weak association with the risk of CP. A longer time spent with a viral load of 0 to 500 copies/mL (RR = 0.63, 95% CI: 0.51 to 0.77; P = 0.0001; see Fig. 2B) or even with a viral load of 501 to 10,000 copies/mL (RR = 0.60, 95% CI: 0.39 to 0.93; P = 0.02) was associated with a reduced risk of CP.
As to the adjusted RRs associated with viral suppression compared with the set point, there was evidence for an increased risk of progression in patients with a current suppression between >0 and 1.5 log10 copies/mL (RR = 2.34, 95% CI:1.16 to 4.74; P = 0.02) and in those with no suppression or a viral load higher than their set point (RR = 2.39, 95% CI:1.17 to 4.89; P = 0.02; see Fig. 3A) compared with those with suppression >3 log10 copies/mL. Again, we found strong evidence for the existence of a linear trend when we fit the variable as an ordinal (RR = 1.52 per unit higher, 95% CI: 1.20 to 1.92; P = 0.0005). Longer duration on HAART with a viral load suppressed to less than the set point seemed to confer protection against AIDS and HIV-related death (35% reduction in risk per year longer on average; see Fig. 3B).
Other factors associated with the risk of CP in this analysis were the CD4 nadir (RR = 0.81 per 100 cells/mL higher, 95% CI: 0.71 to 0.92; P = 0.002) and CDC stage (RR = 1.64 for stage B vs. stage A, 95% CI: 1.04 to 2.58; P = 0.03 and RR = 2.59 for stage C vs. stage A, 95% CI: 1.58 to 4.25; P = 0.0002).
When we repeated the analysis after having excluded events and person-years of patients for whom the viral load had been measured less than twice per year, the results were similar. The overall rate of CP was slightly lower than the estimate of the main analysis: 88 events during 9125 PYFU (9.6 per 1000 PYFU, 95% CI: 7.7 to 11.9). The results for the comparisons of interest were similar, however: the RR per year spent on HAART with a viral load >100,000 copies/mL was 1.81 (95% CI: 0.95 to 3.46), and it was 0.69 (95% CI: 0.34 to 1.40) for time on a viral load of 10,001 to 100,000 copies/mL, whereas this risk was significantly <1 for the other viral load strata (RR = 0.37, 95% CI: 0.18 to 0.79 for time on a viral load of 501-10,000 copies/mL and RR = 0.51, 95% CI: 0.38 to 0.67 for time on a viral load suppressed to less than 500 copies/mL). Similarly, any time spent with a viral load suppressed lower than the set point seemed to confer clinical benefit (RR = 0.51, 95% CI: 0.34 to 0.76 per year longer with a suppression >3 log copies/mL; RR = 0.56, 95% CI: 0.39 to 0.81 per year longer with a suppression of 1.5-3.0 log copies/mL; and RR = 0.49, 95% CI: 0.28 to 0.85 per year longer with a suppression of 0-1.5 log copies/mL).
Drug Resistance Analysis
Only 2357 PYFU of the total 11,447 PYFU (20.6%) over the period starting from the dates of the first available genotypic test to the date of last clinical follow-up were used in this subanalysis. It should be noted that, as expected, patients with a genotypic test were more likely to be patients who had experienced virologic failure. Patients with a genotypic test were not different from those who did not have a genotypic test with respect to a number of general characteristics, however: female gender (26.3% vs. 29.4%; P = 0.14), age (median: 36 vs. 35 years; P = 0.94), and mode of HIV transmission (injection drug user [IDU]: 31.9% vs. 35.8%, homosexual: 23.9% vs. 19.7%, and heterosexual: 38.0% vs. 37.8%; P = 0.11).
We found that there was no evidence of accumulation of resistance for 1487 PYFU (63.1%) and that there was evidence of accumulation of resistance to 1 drug class for 501 PYFU (21.3%), to 2 drug classes for 315 PYFU (13.4%), and to all 3 drug classes for 53 PYFU (2.3%). These proportions were similar across groups stratified by the current viral load level; however, a significantly higher proportion of PYFU spent with resistance to 3 drug classes was in the stratum of >100,000 copies/mL (10.1% vs. average of 3.2%; P = 0.0001).
DISCUSSION
In our analysis, 9.9% of the total person-years of therapy with HAART (1130 of 8778 PYFU) were spent with moderate viremia (between 501 and 10,000 copies/mL). We found no evidence that a longer duration of time with such moderate viremia was associated with a higher risk of subsequent CP, however.
Current guidelines recommend a switch of therapy as soon as virologic failure is confirmed, and complete viral suppression remains the ultimate goal of therapy, especially in antiretroviral-naive people.1 If it is possible to construct a new regimen that is potent virologically (eg, contains ≥3 active drugs) and is expected to be easily accepted by the patient, this is certainly the recommended strategy. This is not always possible, however; some patients have limited options because they carry a multiresistant virus or are intolerant to specific drugs.
Our study and several other reports4-7 offer different arguments in favor of keeping patients on failing regimens and delaying the switch to when more active drugs are available. First of all, CD4 cells may remain stable or even increase in some patients taking HAART despite the persistence of low but detectable viremia4,5,14,15 as long as the viral load is suppressed to less than the natural set point6 or despite a rebound after an initial virologic response.16,17 A viral load rise in patients with a sustained virologic failure between 1000 and 10,000 copies/mL and continuing the same failing HAART was estimated to be only 0.024 log10 copies/mL per month, with little impact on the slope of the CD4 count.6 The maintenance of a stable CD4 count is important, because in patients without complete virologic suppression when taking HAART, the risk of CP is strongly correlated to this marker.14 In a recently published article, Deeks et al8 observed a complex, curvilinear, bell-shaped association between the steady-state viral load and the frequency of virus-specific CD4+ T cells (as assessed by measurement of levels of Gag-specific CD4+ T-cell responses); only at high levels of viremia (>3.3 log10 copies of RNA per milliliter) was an increase in the level of viral replication associated with a decrease in the HIV-specific CD4+ T-cell response.
The preservation of a stable CD4 count or of a low risk of CP despite persistent HIV viremia may be attributable to different mechanisms, including diminished fitness of mutant viruses potentially associated with a decreased cytopathic effect,8,18 inability of mutant strains of HIV to replicate in the human thymus,19 decreased T-cell apoptosis,20 and stimulation of HIV-specific CD8 T cells by exposure to HIV antigens.21
Even though the relation between viral replication and immune reconstitution is probably linked in a complicated and nonlinear manner, it may be possible to hypothesize that if a patient's viral load is kept below a certain threshold, there would potentially only be the risk of drug resistance accumulation without an immediate increase in CP. In a study by Deeks at al,4 there was no decline in CD4 count in people who failed to maintain undetectable HIV viremia; rather, it remained less than 30,000 copies/mL. Le Moing et al17 identified an HIV viremia threshold of 5000 to 10,000 copies/mL, below which the risk of immunologic failure is not significantly increased. In a large intercohort analysis in people who had experienced 3-class virologic failure and continue to take HAART,7 viremia less than 10,000 copies/mL or suppression at least 1.5 log copies/mL less than the pretherapy value was not associated with a decline in CD4 cell count.
Our study focused not only on the association between the current viral load (or viral load suppression relative to the set point) and CP but on that between the duration of time spent in these viral load categories and progression. A longer time spent with a viral load between 501 and 100,000 copies/mL does not seem to be detrimental to the future clinical prognosis of our patients. Only a longer time spent with a viral load >100,000 copies/mL seemed to be associated with an increase in the risk of future CP, although the risk was not statistically significant (22% increase in risk per year longer, P = 0.25). When we looked at the current viral suppression relative to patient's natural set point to identify a threshold that could be useful for patients who have a viral load measured before ART initiation or while off therapy, however, we found that those who currently had viral suppression less than 1.5 log10 relative to the set point tended to have an increased risk of CP compared with those with viral suppression >1.5 log10 (see Fig. 3A). This result is in agreement with the result of another study,7 and it suggests that the 1.5-log suppression may be a crucial threshold. Interestingly, however, as long as suppression below the set point was achieved, a longer time spent with such suppression (whatever the level) was associated with a clinical benefit.
The present study has several limitations. First, the median of follow-up was only 3.8 years. It is possible that a higher rate of CP associated with the duration of time spent with a viral load ranging between 501 and 100,000 copies/mL may become apparent with longer follow-up.
Second, only limited data on the accumulation of drug resistance mutations over time were available. Thus, based on this study, it is difficult to assess how much damage in terms of the rate of accumulated resistance has been created by keeping patients on failing therapy. Under the pressure of a failing regimen, the probability of accumulating drug resistance mutations is high,22-24 especially at low but detectable viremia,15,25 even though this may partially be in the patient's favor if the accumulated mutational pattern is associated with a reduction in HIV replicative capacity. For example, the selection of 184V seems to resensitize HIV partially to thymidine analogues.26 In a large cohort study on the survival of HIV-infected people, the prevalence of multidrug resistance was relatively low, indicating that the exhaustion of treatment options because of drug resistance was not a significant contributor to mortality.27
Conversely, it has been shown that the replicative capacity of PI-resistant variants may be restored over time by the acquisition of compensatory mutations.28 Moreover, the stable number of CD4 cells observed in people with low but detectable viremia may provide a larger pool of susceptible cells reinforcing a detectable burst of viral replication (predator/prey phenomenon).29 Then, it would be interesting to know from future studies whether specific patterns of mutations that confer reduced replicative capacity are associated with slower increase of viral load.
Third, we cannot exclude the possibility that our data could be influenced by a large variability in adherence to HIV therapy guidelines and by an awareness of the consequences of moderate viremia by clinicians managing patients at the different sites. Furthermore, there may be specific reasons why clinicians and/or patients decide to delay a therapy switch that are not entirely captured by our analysis. The reasons for deferring a therapy switch in different settings should be further investigated, possibly in a separate study.
Virologic suppression to less than 50 copies/mL is not always achieved in patients treated with HAART. Even if we did not find, in the short term, an excess of risk of CP in patients who retained a viral load between 501 and 100,000 copies/mL for a longer period or with viral suppression not greater than 1.5 log10 compared with the viral set point, on the basis of this finding alone, we cannot recommend a deferred therapy switch in people with virologic failure.
In case of virologic failure, when at least 3 active antiretroviral drugs are still available, switching therapy should be considered the main strategy.
Only prospective clinical trials that randomize multidrug-experienced patients with virologic failure and few remaining options to an immediate or delayed therapy switch may help to determine the feasibility of this drug conservation strategy. In patients who have exhausted all treatment options, continuing a virologically failing therapy rather than waiting for the availability of new therapeutic options seems to be a better strategy than interrupting. Indeed, it has been shown that therapy interruption is associated with a high immediate risk of CP,30 whereas continued therapy, especially with a PI, seems to be associated with protection against opportunistic infections over and above that conferred by the increase in CD4 count and decrease in viral load alone.9 Moreover, Deeks et al31 have shown that in patients with detectable viremia, an immunologic deterioration may occur but only after a prolonged period (3 years).
In conclusion, virologic failure to antiretroviral drugs is common even in patients starting HAART when they are antiretroviral naive. Current treatment guidelines, mainly because of the risk of drug resistance accumulation in patients receiving virologically failing regimens, recommend that treatment should be modified shortly after virologic failure. This seems to be the best available strategy in patients who are likely to achieve viral suppression. Nevertheless, our data suggest that in patients who are kept on nonfully suppressive regimens, the risk of subsequent CP may remain low despite a low but detectable level of HIV viremia. These data need to be re-evaluated at a later stage when a longer follow-up interval is available to confirm whether a longer duration of time with a viral load ranging between 501 and 100,000 copies/mL is still not associated with a remarkable increase in the risk of CP. Further studies, possibly with a longer follow-up interval than ours and a larger number of events, are warranted.
PATIENTS AND METHODS
We studied participants in the ICoNA study, a large observational study started in 1997 in 69 Italian infectious disease wards in which patients were enrolled when still antiretroviral naive, independent of the reason for not having started therapy.11 Inclusion criteria for this analysis were the following: having started HAART, having been followed for at least 6 months, and having an average of at least 2 HIV viral load measurements per year of follow-up. The follow-up interval was defined as the time from the date of enrollment to the date of the most recent clinical visit. All the data reported in the database up to May 15, 2005 were used for this analysis.
The study outcome was CP, a composite end point of death and disease progression. A new AIDS diagnosis was defined as the time at which a person was diagnosed with one of the AIDS-defining diseases listed in the 1993 Centers for Disease Control and Prevention (CDC) case definition12 if this illness had not been diagnosed previously. The time of the event was assigned as the time of a new AIDS diagnosis or death. AIDS events that occurred after this date have been ignored. The follow-up of patients who did not develop any of these events was censored at the date of the last clinical visit.
Time 0 of the analysis was 6 months after the date of HAART initiation. The rationale for this choice was that until this point in time, a day spent without complete viral suppression could not be considered the same as one that occurs after 6 months of therapy initiation, because not enough time would have passed to verify whether the therapy had or did not have an effect on viral replication. Starting from the day after month 6, we counted, for each patient, the cumulative number of person-years spent in each of the following categories: non-HAART (including monotherapy and dual therapy as well as periods of interruptions), on HAART with a viral load <500 copies/mL, on HAART with a viral load between 501 and 10,000 copies/mL, on HAART with a viral load between 10,001 and 100,000 copies/mL, and on HAART with a viral load >100,000 copies/mL. A cutoff of 10,000 copies/mL was chosen based on the previous observation that clinical and immunologic benefits were maintained in patients with a viral load fluctuating between 200 and 10,000 copies,3,7 whereas the cutoff of 100,000 copies/mL was chosen to test whether this finding could be extended to individuals with a viral load fluctuating between even higher levels. In alternative analyses, instead of using the absolute viral load levels and these cutoffs, we divided the person-years (and events) according to the change in viral load from the most recent value before initiation of antiretroviral therapy (ART; patient's natural viral set point [ie, maximum level of replication for each individual]). In particular, we grouped the person-years in which the viral load was the same or higher than the set point, with a suppression between 0.1 and 1.5 log, with a suppression between 1.51 and 3 log, and with a suppression >3 log below the set point.
In addition, the risk of CP associated with the most recent level of viremia (or change from the set point) was estimated. The same cutoffs were chosen for these time-updated covariates.
A multivariable Poisson regression model was constructed, including a number of covariates that had been chosen a priori on the basis of being potential confounders for the association of interest (duration of viral load suppression in predefined viral load strata and CP). Some of these variables were fixed factors (age, gender, mode of HIV transmission, CD4 nadir before ART initiation, calendar year of HAART initiation, clinical stage at entry, whether the patient had been hospitalized before enrollment or not, and use of opportunistic infection prophylaxis before ART initiation), and others were time-dependent factors (current viral load fit as a categoric variable, duration of time spent in each viral load stratum, cumulative number of drugs started during follow-up, current use of nonnucleoside reverse transcriptase inhibitor [NNRTI] or PI, and current hepatitis C virus [HCV] serology). The duration of time spent in each viral load stratum was modeled in a linear fashion. In particular, the covariate indicating such duration was expressed in year and fit in the Poisson regression as a continuous variable, such that the relative risk (RR) would be expressing the risk of CP associated with each additional year spent in a particular viral load stratum.
Two separate models were fit: the first with the absolute values of the viral load and the second with the viral load suppression from the natural set point (both fit as categoric variables with cutoff as described previously). The rate associated with a current category of viral load and that associated with longer time spent in the same viral load category were estimated in each model.
Resistance Data
Patients were defined as carrying a virus that was resistant to a class of drugs if ≥1 mutation associated with resistance to that drug class (according to 2005 International AIDS Society [IAS] definition)13 was detected in their genotypic sequence. Once a mutation was detected, we assumed that such a patient would retain a virus with that mutation from that date onward. The time 0 for this analysis was the date at which the first genotypic test was performed, and follow-up was truncated at the date of last clinical visit. The proportion of follow-up with viruses carrying resistance was calculated by dividing the amount of time during which patients were defined to carry resistance to 1, 2, or 3 drug classes for the total person-years of observation.
Resistance testing in the cohort has been performed in a random sample of patients who had a stored plasma sample before initiation of combination therapy. For a subset of these patients, for whom a stored plasma sample at virologic failure of the first regimen was also available, a second genotypic test was performed.
|
|
| |
| |
|
|
|