| |
Renal Safety: TDF + 3TC/EFV (144 wks, Phase 3 Study 903)
|
| |
| |
Reported by Jules Levin
Similar Renal Safety Profile Between Tenofovir DF (TDF) and Stavudine (d4T) Using Modification of Diet in Renal Disease (MDRD) and Cockroft-Gault (CG) Estimation of Glomerular Filtration Rate (GFR) in ART-Naive Patients Through 144 Weeks: Study 903- TDF vs d4T + 3TC & Efavirenz
JE Gallant1, S Staszewski2, AL Pozniak3, E DeJesus4, B Lu5, J Enejosa5, and A Cheng5 for the 903 Study Team
1Johns Hopkins Univ. School of Medicine, Baltimore, Maryland, USA; 2University Hospital, J.W. Goethe-Universitat, Frankfurt, Germany; 3Chelsea and Westminster Hosp, London, UK; 4 Orlando
Immunology Center, Orlando, Florida, USA; 5Gilead Sciences, Inc., Foster City, California, USA
45th Interscience Conference on Antimicrobial Agents and Chemotherapy
December 16-19, 2005, Washington, D.C., USA
Poster Number H-350
Background
Both the Cockcroft-Gault (CG) formula and the Modification of Diet in Renal Disease (MDRD) equation are used to estimate creatinine clearance and glomerular filtration rate (GFR). They provide a better measure of renal function than serum creatinine alone, but the MDRD appears to be more accurate in HIV-negative patients with chronic kidney disease1
Estimation of GFR using MDRD has not been validated in patients with normal kidney function or in HIV-infected patients1
Author Conclusions
- The renal safety profile was similar between patients receiving TDF or d4T in combination with 3TC and EFV through three years of treatment
- No patient developed Proximal Renal Tubular Dysfunction and/or Fanconi Syndrome through 144 weeks
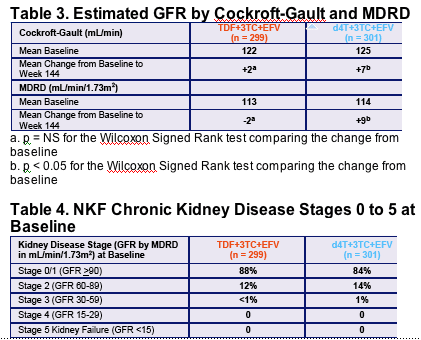
- There were no significant changes in GFR by either MDRD or CG equations from baseline to week 144 in the TDF arm
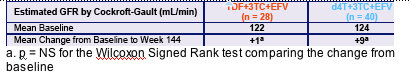
- Among patients in the TDF arm who are also taking concomitant antihypertensives and/or diabetes medications, no significant changes in GFR by CG from baseline to week 144 were observed
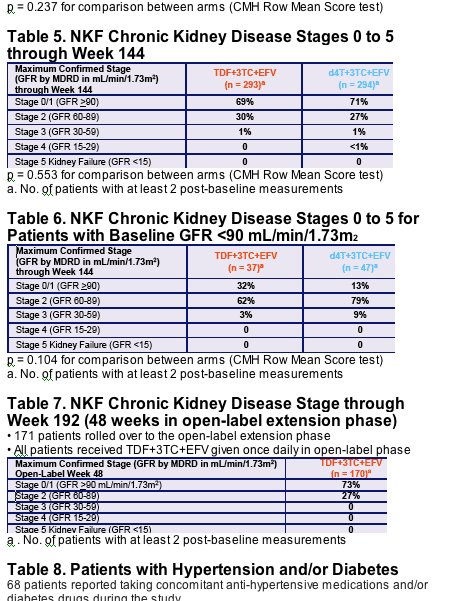
- The incidence of NKF chronic kidney disease stages was similar between TDF and d4T through 144 weeks
Methods
Study 903 is a Phase III trial with an ongoing open-label extension phase
and a completed 144-week, randomized, double-blind phase designed to
evaluate TDF vs. d4T in combination with lamivudine (3TC) and efavirenz
(EFV) in treatment-naive patients
We evaluated GFR (estimated by MDRD and CG) and the incidence of
National Kidney Foundation (NKF) Stage 0 to 5 chronic kidney disease
through 144 weeks
Main Inclusion Criteria
- HIV-1 infected patients naive to antiretroviral treatment
- 18-65 years of age
- Plasma HIV RNA > 5,000 copies/mL
- No CD4+ cell count criteria
- No significant laboratory or clinical abnormalities
- Serum Creatinine < 1.5 mg/dL
- Serum Phosphorus > 2.2 mg/dL
-- Estimated GFR by Cockroft-Gault > 60 mL/min
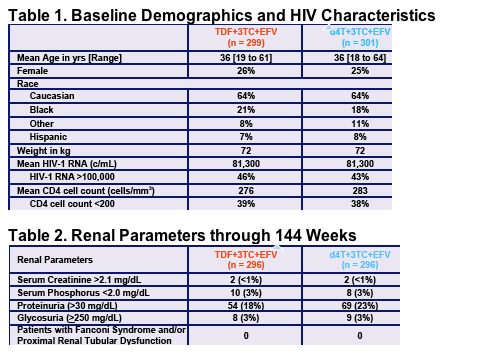
Renal Adverse Events
- No patient discontinued due to tenofovir DF-related renal abnormalities through 144 weeks
- No patient developed Proximal Renal Tubular Dysfunction and/or Fanconi Syndrome through 144 weeks
- Proximal Renal Tubular Dysfunction (PRTD) was defined as:
- Confirmed rise in serum creatinine of > 0.5 mg/dL from baseline AND serum phosphorus <2.0 mg/dL, OR
- Either serum creatinine of > 0.5 mg/dL from baseline or serum phosphorus <2.0 mg/dL, accompanied by any two of the
following:
- Proteinuria (>100 mg/dL)
- Glycosuria (>250 mg/dL)
- Low serum potassium (<3 mEq/L)
- Low serum bicarbonate (<19 mEq/L)
References
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39:1-266.
|
|
| |
| |
|
|
|