| |
Tenofovir-Associated Acute and Chronic Kidney Disease: A Case of Multiple Drug Interactions
|
| |
| |
Clinical Infectious Diseases Jan 14, 2006;42:283-290
Anthony E. Zimmermann,1,2 Thomas Pizzoferrato,2 John Bedford,3 Anne Morris,3,4 Robert Hoffman,2 and Gregory Braden3,4
1Massachusetts College of Pharmacy and Health Science, Worcester, 2Mercy Medical Center and 3Baystate Medical Center, Springfield, and 4Tufts University School of Medicine, Boston, Massachusetts
".....renal function (including determination of blood urea nitrogen, serum creatinine, electrolyte, calcium, phosphorus, and magnesium levels) should be monitored every 2 weeks for the first 2 months of treatment, then monthly thereafter, for patients who are receiving tenofovir concomitantly with ritonavir or lopinavir-ritonavir, ritonavir plus didanosine, or ritonavir plus atazanavir....."
ABSTRACT
Tenofovir therapy in patients with human immunodeficiency virus (HIV) infection has been associated with acute renal failure (ARF) and Fanconi syndrome. In the past 2 years, we diagnosed tenofovir-associated ARF in 5 HIV-infected patients who were receiving tenofovir therapy and who had classic findings of acute tubular necrosis, and we compared findings for our patients with data on 22 patients described in the literature. The mean serum creatinine level increased from 0.9 to 3.9 mg/dL, and it decreased to 1.2 mg/dL during recovery. ARF resolved in 22 of 27 patients after discontinuation of tenofovir therapy. The most common drugs given with tenofovir were ritonavir or lopinavir-ritonavir (21 of 27 patients), atazanavir (5 of 27 patients), and didanosine (9 of 27 patients). Tenofovir-associated ARF manifests as acute tubular necrosis that may not resolve with tenofovir withdrawal. Tenofovir is associated with multiple drug interactions, leading to an increased risk of ARF. Frequent monitoring of renal function is warranted for any patient receiving these combinations.
INTRODUCTION
Patients with HIV infection are at increased risk of drug-induced renal toxicity, most commonly associated with trimethoprim-sulfamethoxazole (TMP-SMZ), pentamidine, or acyclovir treatment. Acute renal failure (ARF) has also been occasionally associated with receipt of indinavir, ritonavir, adefovir, and cidofovir [15]. Tenofovir (Viread; Gilead) is a new nucleotide reverse-transcriptase inhibitor used with other antiretroviral agents for the treatment of HIV infection.
We report the diagnosis of tenofovir-associated ARF in 5 patients during the past 2 years who had classic acute tubular necrosis. Acute tubular necrosis was identified by urinary sediment with pigmented granular casts or in renal biopsy specimens, examination of which revealed the unique lesions due to karyomegaly in proximal tubular nuclei. Incomplete recovery of renal function occurred in 3 patients. We report the clinical features of our 5 patients, as well as those of 22 patients described in the medical literature. We suggest a possible mechanism for ARF in these patients and recommend monitoring guidelines.
METHODS
A literature search for published case reports involving tenofovir and ARF was performed using the Medline database (for articles published from 1990 through January 2005), with the following search terms: "tenofovir," "renal lesions," "acute renal failure," "Fanconi's syndrome," and "tubular dysfunction." Twenty-two cases of tenofovir-related ARF were identified [614]. Complete patient medical and medication histories were obtained from our 5 patients. Pertinent laboratory tests were performed to exclude other causes of ARF; tests included renal ultrasound, antinuclear antibodies, antineutrophil cytoplasmic antibodies, C3 and C4 complement, rapid plasma reagin, and determination of antistreptolysin O titers (performed for all patients), as well as renal biopsy (performed for 1 patient). The duration of follow-up for the 5 patients was 220 months.
The glomerular filtration rate was calculated on the basis of the 24-h creatinine clearance rate and/or the Cockroft-Gault formula. Fanconi syndrome was characterized by abnormalities in proximal renal tubular function resulting in glycosuria, with normal serum glucose levels, phosphaturia, aminoaciduria, and decreased serum bicarbonate levels [15]. Statistical analysis was performed using Student's t test for paired and unpaired data. A P value of <.05 was considered to be statistically significant. Baseline data and clinical characteristics for all 27 patients are shown in tables 1 and 2.
CASE REPORTS
Patient 1. A 61-year-old man with a baseline serum creatinine level of 1.0 mg/dL and creatinine clearance rate of 74 mL/min was admitted to the hospital with ARF. Past medical history was significant for AIDS of 4 years' duration and chronic hepatitis B, with normal liver function test results prior to and at admission and normal abdominal CT findings prior to admission. Medications received included atorvastatin, omeprazole, quetiapine, oxcarbazepine, and prophylaxis with azithromycin and TMP-SMZ. His HAART regimen consisted of saquinavir (1000 mg twice per day), didanosine (400 mg per day), lopinavir-ritonavir (400/100 mg twice per day), and tenofovir (300 mg per day) for the past 16 months. Two months before hospital admission, the patient's serum creatinine level had increased to 1.4 mg/dL, but none of the components of his HAART regimen were adjusted on the basis of his renal function. Admission laboratory tests revealed the following concentrations: sodium, 119 mmol/L; potassium, 3.5 mmol/L; bicarbonate, 17.5 mmol/L; serum creatinine, 2.8 mg/dL; glomerular filtration rate, 17 mL/min; blood urea nitrogen, 43 mg/dL; uric acid, 2.8 mg/dL; and phosphorus, 2.2 mg/dL. Urinalysis revealed a protein level of 100 mg/dL, a glucose level of 500 mg/dL, and sheets of pigmented granular casts. The findings of renal ultrasound were normal, as were the findings for all other serologic tests. Tenofovir therapy was discontinued. The patient was discharged from the hospital receiving lopinavir-ritonavir, saquinavir, and renally adjusted doses of TMP-SMZ, didanosine, and lamivudine. Twenty months later, renal function had failed to return to the baseline level, with a serum creatinine level of 2.6 mg/dL and creatinine clearance rate of 19 mL/min, but without proteinuria on urinalysis.
Patient 2. A 53-year-old man with AIDS of 8 years' duration and hepatitis C, with normal liver function test results and abdominal CT findings, was admitted to the hospital with acute respiratory distress, pneumonia, and ARF. For the past 20 months, he had been treated with lopinavir-ritonavir (400/100 mg twice per day), tenofovir (300 mg per day), and stavudine (40 mg twice per day). The baseline serum creatinine level was normal (0.8 mg/dL), and the glomerular filtration rate was 94 mL/min. Six weeks before hospital admission, the patient's serum creatinine level had increased to 1.8 mg/dL, but no adjustments had been made to his HAART regimen. At hospital admission, laboratory tests revealed the following concentrations: sodium, 131 mmol/L; potassium, 3.7 mmol/L; chloride, 99 mmol/L; bicarbonate, 12.2 mmol/L; blood urea nitrogen, 91 mg/dL; creatinine, 7.4 mg/dL (with a glomerular filtration rate of 10 mL/min); calcium, 7.4 mg/dL; phosphorus, 5.4 mg/dL; and albumin, 2.5 mg/dL. Urinalysis revealed 1+ protein, 2+ blood, and many pigmented granular casts. The findings of all other laboratory tests and of an ultrasound were normal. Tenofovir treatment was discontinued. The patient restarted lopinavir-ritonavir treatment and renally adjusted doses of lamivudine and stavudine. Four months later, tenofovir was inadvertently readministered at another hospital for a total of 10 days. His serum creatinine level increased to 1.8 mg/dL from his initial discharge level of 1.2 mg/dL. Tenofovir treatment was discontinued again, and after 1 month, his serum creatinine level decreased to 1.4 mg/dL. At the 10-month follow-up visit, the patient's creatinine level was still 1.4 mg/dL, and the glomerular filtration rate was 37 mL/min, without proteinuria.
Patient 3. A 48-year-old man with AIDS of 11 years' duration and hepatitis C, with normal liver function test results, presented with ARF and hyperkalemia. The baseline serum creatinine level was 1.0 mg/dL, and the glomerular filtration rate was 90 mL/min. He had been receiving tenofovir (300 mg per day), lamivudine (150 mg twice per day), saquinavir (600 mg twice per day), and lopinavir-ritonavir (400/100 mg twice per day) for 12 months, as well as TMP-SMZ (1 tablet [160 mg and 800 mg] 3 times per week) and clarithromycin (500 mg twice per week). Initial laboratory studies revealed the following concentrations: blood urea nitrogen, 53 mg/dL; creatinine, 1.8 mg/dL (with a glomerular filtration rate of 53 mL/min); sodium, 140 mmol/L; potassium, 5.6 mmol/L; chloride, 103 mmol/L; and bicarbonate, 24 mmol/L. Urinalysis revealed pigmented granular casts. A 24-h urine collection reported 0.65 g of protein per day or moderate proteinuria. Serologic test and renal ultrasound findings were normal. Tenofovir treatment was discontinued. At discharge from the hospital, the patient restarted receiving all previous medications except tenofovir. After 2 months, his blood urea nitrogen level was 21 mg/dL, and his serum creatinine level was 0.8 mg/dL, with a creatinine clearance rate of 97 mL/min.
Patient 4. A 65-year-old man with AIDS of 8 years' duration and type II diabetes mellitus was admitted to the hospital with ARF. The baseline serum creatinine level was 0.7 mg/dL, and the glomerular filtration rate was 68 mL/min. The medications he had been receiving included tenofovir (300 mg per day), nevirapine (200 mg twice per day), lopinavir-ritonavir (400/100 mg twice per day), lamivudine (150 mg per day), glipizide, and fenofibrate for 24 months. Admission laboratory tests revealed the following concentrations: blood urea nitrogen, 68 mg/dL; creatinine, 7.1 mg/dL (with a glomerular filtration rate of only 6.8 mL/min); sodium, 140 mmol/L; potassium, 3.9 mmol/L; chloride, 115 mmol/L; and bicarbonate, 10 mmol/L. Urinalysis revealed many pigmented granular casts. A renal biopsy was performed, the results of which are shown in figure 1. The patient received 2 hemodialysis treatments for azotemia. Tenofovir treatment was discontinued. He restarted receiving all previous medications except tenofovir. Twelve months later, his serum creatinine level was 0.8 mg/dL, and his glomerular filtration rate was 60 mL/min.
RESULTS
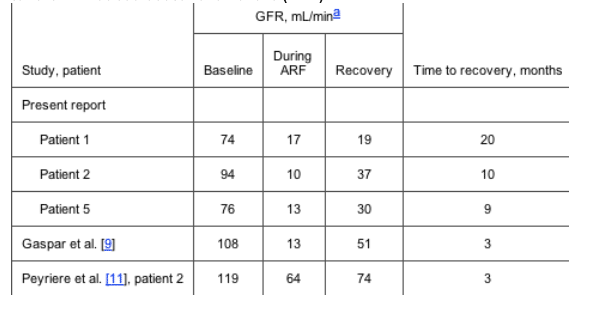
We analyzed the findings for our 5 patients and for the 22 patients described in the literature with tenofovir-associated ARF since December 2002. The mean age of the 27 patients was 45.5 years (range, 3165 years), with a ratio of men to women of 3.5 : 1. The mean duration of tenofovir therapy was 11 months (range, 129 months). The drugs most often administered with tenofovir were ritonavir or lopinavir-ritonavir (21 of 27 patients), didanosine (9 of 27 patients), and atazanavir (5 of 27 patients). It should be noted that some didanosine doses (due to renal impairment) and atazanavir doses (when used in combination with other agents) were not appropriate. The mean serum creatinine level increased from 0.9 to 3.9 mg/dL (P < .05), and it decreased to 1.2 mg/dL during recovery (P < .05). Tenofovir treatment was discontinued for all patients, and abnormal laboratory findings dramatically improved or resolved in the majority of patients. However, the glomerular filtration rate decreased from a mean of 81 to 31 mL/min and increased to 59 mL/min during recovery in a subset of 12 of the 27 patients. The glomerular filtration rate in 5 patients did not return to the baseline level after a mean duration of follow-up of 7.5 months (range, 320 months) (table 3). Two patients required temporary hemodialysis. Examination of renal biopsy specimens obtained from 8 patients revealed acute tubular necrosis, with nuclear swelling and karyomegaly of proximal tubular nuclei. Sixteen patients had Fanconi syndrome, 19 had nonanion gap metabolic acidosis, 13 had hypokalemia, and 5 had nephrogenic diabetes insipidus. After discontinuation of tenofovir treatment, Fanconi syndrome resolved in all patients, and renal function returned to the baseline level in 22 of the 27 patients. We attempted to correlate the patients' viral loads and CD4 cell counts with the development of ARF, but not enough case reports included that information.
Table 3. Characteristics of patients with incomplete recovery from tenofovir-induced acute renal failure (ARF).
DISCUSSION
Tenofovir represents the first of a new class of antiretrovirals, the nucleotide reverse-transcriptase inhibitors. Two similar acyclic nucleoside phosphonate antiviral derivatives, adefovir and cidofovir, have been associated with dose-limiting, renal tubular cell toxicity in patients with infectious hepatitis or cytomegalovirus infection who have been treated with these agents [13]. Proposed mechanisms for this drug-induced proximal tubular toxicity include epithelial cell mitochondrial DNA depletion [1, 2] and/or direct tubular cytotoxicity [17]. Unlike these 2 agents, no direct association with mitochondrial toxicity has been found for tenofovir [18]. Proximal tubular accumulation of all these drugs occurs by direct transport by the human organic anion transporter 1 (hOAT1) on the basolateral side of the proximal renal tubular cells.
We believe that multiple drug interactions with tenofovir and other HIV drugs lead to renal tubular toxicity and tenofovir-associated ARF. Tenofovir predominately accumulates in proximal renal tubular cells and is eliminated by active tubular secretion and glomerular filtration. The renal clearance of tenofovir in humans is significantly greater than the glomerular filtration rate, indicating that renal tubular secretion of tenofovir occurs. Active uptake of nucleotides from blood into proximal tubular cells occurs via hOAT1, which is located in the basolateral membrane of proximal tubules [17]. Once accumulated, the nucleotides are secreted into the urine via the multidrug-resistance protein (MRP2) on the apical side of the proximal tubular cell. The package insert states that the dose of tenofovir should be adjusted for patients with a creatinine clearance rate of <50 mL/min [19]. If the dose is not adjusted, the increased tenofovir concentrations could increase the possibility of developing tenofovir-associated ARF.
In addition, administration of ritonavir alone or with lopinavir has been shown to increase the maximum serum concentrations of tenofovir by >30% [20]. Ritonavir is not an inhibitor of hOAT1 but is a potent inhibitor of MRP2-mediated transport, which transports anionic compounds, including tenofovir [21]. It is also an inhibitor of P-glycoprotein, an efflux pump for organic cations. We believe that it is likely that ritonavir increased proximal tubular concentrations of tenofovir by decreasing urinary secretion through this pathway, because 21 of 27 patients with ARF or Fanconi syndrome were receiving ritonavir alone or in combination with lopinavir.
Didanosine is eliminated by glomerular filtration and active tubular secretion [22]. The renal clearance of didanosine is significantly greater than the glomerular filtration rate, indicating that renal tubular secretion of didanosine occurs. Compared with patients who have normal renal function, in patients with chronic renal failure, there are significant increases in the half-life and significant decreases in the total body clearance of didanosine [23]. An early case report by Crowther et al. [24] from 1993 described the first case of possible didanosine-induced Fanconi syndrome and nephrogenic diabetes insipidus. Coadministration of tenofovir with didanosine has resulted in a significant increase (28%) in maximum serum concentrations of didanosine, leading to an increased risk of didanosine toxicity [19]. Didanosine is taken up by hOAT1 at the proximal tubules, and it is possible that competition between tenofovir and didanosine for the hOAT1 transporter produces an increase in the didanosine concentration, leading to an increased risk of mitochondrial damage and nephropathy. Ten of the 27 reported patients received tenofovir and didanosine. A reduction in the dosage of didanosine is recommended when it is coadministered with tenofovir [19].
In in vitro studies, atazanavir has been shown to be an inhibitor and inducer of P-glycoprotein and an inhibitor of cytochrome P450 3A activity [25]. The pharmacokinetic studies of atazanavir coadministered with ritonavir have reported increases in both the maximum serum concentration (34%) and the area under the curve (41%) of ritonavir [26]. Coadministration of tenofovir with atazanavir resulted in increases in the following tenofovir pharmacokinetic parameters: maximum concentration, 14%; minimum concentration, 22%; and area under the curve, 24%. Likewise, when coadministered with tenofovir, atazanavir use has resulted in a decrease (26%) in the area under the curve of atazanavir [19, 27]. The exact mechanism for this drug interaction is not known, but 5 of the 27 patients in our study with ARF were receiving 300-400 mg atazanavir daily. Patients who were receiving both ritonavir and atazanavir should be carefully monitored for an increase in tenofovir-associated adverse effects [19].
The safety profile of tenofovir has been reported to be safe and is similar to that of placebo [28]. However, several recent case reports of drug-induced renal tubular dysfunction and Fanconi syndrome involving patients who had been taking tenofovir for up to 26 months have been published [7, 10, 13, 14]. Verhelst et al. [14] and Karras et al. [10] reported cases of tenofovir-induced tubular injury with Fanconi syndrome in HIV-infected patients who had normal renal function. These patients developed tubular injury 1 to >26 months after initiating tenofovir treatment. Another case of renal tubular dysfunction was reported to have occurred in a patient with stable chronic renal disease [7]. Schaaf et al. [13] described a patient who presented with proximal tubular necrosis without Fanconi syndrome after only 8 weeks of tenofovir therapy.
In all cases, CD4 cell count and plasma HIV load were not predictors of which patients would develop ARF associated with tenofovir, and Fanconi syndrome resolved upon discontinuation of tenofovir. All other drugs were either continued or restarted after resolution of the acute event, with no further deterioration of renal function. Other causes of AIDS-related or drug-induced renal insufficiency were ruled out. No evidence of tubulopathy was present prior to this acute event. We have now observed our patients after ARF for up to 20 months. Three of our 5 patients have had incomplete recovery of glomerular filtration rate, as did 2 other patients described in the literature (table 3), which was not the case for 5 patients recently described in the literature with a 3-month follow-up period [16]. Incomplete recovery of glomerular filtration rate occurred in about 19% of patients who were reported to have ARF associated with tenofovir and ritonavir or lopinavir-ritonavir. To our knowledge, we are the first to report several cases of chronic kidney disease and ARF after discontinuation of tenofovir, with inadvertent rechallenge in 1 patient in whom tenofovir-associated ARF had resolved.
Patients who are taking tenofovir concurrently with either ritonavir, lopinavir-ritonavir, didanosine, or atazanavir should be closely monitored for potentially multiple serious drug-drug interactions leading to acute or chronic kidney disease, Fanconi syndrome, and/or diabetes insipidus. In June 2004, Gilead revised the package insert to include monitoring for adverse effects in patients receiving tenofovir in combination with atazanavir or lopinavir-ritonavir associated with increased tenofovir concentrations, as well as the potential for the development of ARF and Fanconi syndrome.
It is important to remember that many patients may present with muscle wasting while they are receiving HAART. Serum creatinine level is an insensitive measurement of the glomerular filtration rate, and patients could have significant renal insufficiency with normal serum creatinine levels. It is recommended that any change in serum creatinine level of either 0.5 mg/dL or an increase of 50% should alert health care professionals to the potential of renal insufficiency in any tenofovir recipient.
We strongly recommend that renal function (including determination of blood urea nitrogen, serum creatinine, electrolyte, calcium, phosphorus, and magnesium levels) should be monitored every 2 weeks for the first 2 months of treatment, then monthly thereafter, for patients who are receiving tenofovir concomitantly with ritonavir or lopinavir-ritonavir, ritonavir plus didanosine, or ritonavir plus atazanavir. A significant increase in the serum creatinine level or new-onset renal tubular dysfunction during tenofovir therapy with ritonavir, lopinavir-ritonavir, ritonavir plus didanosine, or ritonavir plus atazanavir should lead one to immediately discontinue tenofovir treatment and to perform more-definitive assessments of renal function. Earlier recognition of tenofovir-associated acute changes in renal function will, we hope, prevent the occurrence of chronic kidney disease associated with tenofovir-associated ARF.
References
1. Tanji N, Tanji K, Kambham N, et al. Adefovir nephrotoxicity: Possible role of mitochondrial DNA depletion. Hum Pathol 2001; 32:73440. First citation in article | PubMed | CrossRef
2. Vandercam B, Moreau M, Goffin E, et al. Cidofovir-induced end-stage renal failure. Clin Infect Dis 1999; 29:9489. First citation in article | PubMed
3. Fisher EJ, Chaloner K, Cohn DL, et al. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS 2001; 15:1695700. First citation in article | PubMed | CrossRef
4. Bochet MV, Jacquiaud C, Valantin MA, et al. Renal insufficiency induced by ritonavir in HIV-infected patients. Am J Med 1998; 105:457. First citation in article | PubMed
5. Reiter WJ, Schon-Pernerstorfer H, Dorfinger K, et al. Frequency of urolithiasis in individuals seropositive for human immunodeficiency virus treated with indinavir is higher than previously assumed. J Urol 1999; 161:10824. First citation in article | PubMed | CrossRef
6. Creput C, Gonzalez-Canali G, Hill G, et al. Renal lesions in HIV-1 positive patient treated with tenofovir. AIDS 2003; 17:9357. First citation in article | PubMed | CrossRef
7. Coca S, Perazella MA. Acute renal failure associated with tenofovir: evidence of drug-induced nephrotoxicity. Am J Med Sci 2002; 324:3424. First citation in article | PubMed | CrossRef
8. Dupont C, Meier F, Loupy A, et al. Acute renal failure and tenofovir: 2 new cases [abstract 694]. In: Program and abstracts of the 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment (Paris). Stockholm: International AIDS Society, 2003. First citation in article
9. Gaspar G, Monereo A, Garcia-Reyne A, et al. Fanconi syndrome and acute renal failure in a patient treated with tenofovir: a call for caution. AIDS 2004; 18:3512. First citation in article | PubMed | CrossRef
10. Karras A, Lafaurie M, Furco A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virusinfected patients: three cases of renal failure, Fanconi syndrome and nephrogenic diabetes insipidus. Clin Infect Dis 2003; 36:10703. First citation in article | Full Text | PubMed
11. Peyriere H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy. JAIDS 2004; 35:26973. First citation in article | PubMed
12. Rollot F, Nazal E, Chauvelot-Moachon L, et al. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavir-ritonavir-didanosine. Clin Infect Dis 2003; 37:e1746. First citation in article | Full Text | PubMed
13. Schaaf B, Aries SP, Kramme E, et al. Acute renal failure associate with tenofovir treatment in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 2003; 37:e413. First citation in article | Full Text | PubMed
14. Verhelst D, Monge M, Meynard JL, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis 2002; 40:13313. First citation in article | PubMed
| CrossRef
15. Izzedine H, Launay-Vacher V, Isnard-Bagnis C, et al. Drug-induced Fanconi's syndrome. Am J Kid Dis 2003; 41:292309. First citation in article | PubMed
| CrossRef
16. Rifkin BS, Perazella MA. Tenofovir-associated nephrotoxity: Fanconi syndrome and renal failure. Am J Med 2004; 117:2824. First citation in article | PubMed
| CrossRef
17. Cihlar T, Ho ES, Lin DC, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 2001; 20:6418. First citation in article | PubMed
| CrossRef
18. Birkus G, Hitchcock MJM, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2002; 46(3):71623. First citation in article | PubMed
| CrossRef
19. Viread (tenofovir disoproxil fumarate) [package insert]. Foster City, CA; Gilead Sciences, 2005. First citation in article
20. US Food and Drug Administration. FDA report: background package for NDA 21-356: Viread. 2001. Available at http://www.fda.gov/cder/approval/v.htm. Accessed September 2004. First citation in article
21. Schooley RT, Ruane P, Myers RA, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS 2002; 16:125763. First citation in article | PubMed
| CrossRef
22. Knupp CA, Shyu WC, Dolirf R, et al. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin Pharmacol Ther 1991; 49:52335. First citation in article | PubMed
23. Knupp CA, Hak LJ, Coakley DF, et al. Disposition of didanosine in HIV-seropositive patients with normal renal function or chronic renal failure: influence of hemodialysis and continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 1996; 60:53542. First citation in article | PubMed
| CrossRef
24. Crowther MA, Callaghan W, Hodsman AB, et al. Dideoxyinosine-associated nephrotoxicity. AIDS 1993; 7:1312. First citation in article | PubMed
25. Perloff ES, Duan SX, Skolnik PR, et al. Atazanavir: effects on P-gp transport and CYP3A metabolism in vitro. Drug Metab Dispos 2005; 33(6):76470. First citation in article | PubMed
| CrossRef
26. Boffito M, Kurowski M, Kruse G, et al. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir-boosted once-daily regimen. AIDS 2004; 18:12917. First citation in article | PubMed
| CrossRef
27. Taburet AM, Piketty C, Chazallon C, et al. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2004; 48:20916. First citation in article | PubMed
| CrossRef
28. Guttmann H, Fricker G, Drewe J, et al. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Molecul Pharmacol 1999; 56:3839. First citation in article
|
|
| |
| |
|
|
|