| |
HIV Sexual Transmission Risks: during acute & chronic with <400 c/ml
|
| |
| |
....The major finding of this study was the strong association between increasing serum HIV-1 RNA levels and an increasing risk of heterosexual transmission of HIV-1. In a finding similar to those of studies that found that the risk of perinatal HIV-1 infection is associated with the maternal viral load.....Our data suggest that peripheral-blood levels of HIV-1 RNA contribute dramatically to the risk of heterosexual transmission. Serum HIV-1 RNA levels below 1500 copies per milliliter were not associated with transmission, whereas the risk of transmission increased substantially with increasing viral loads..... For partners with fewer than 400 HIV-1 RNA copies per milliliter, there were zero transmissions....see last of 3 articles below
Amplified HIV Transmission during Early-Stage Infection: rates of transmission per coital act
Journal of Infectious Diseases Feb 15, 2006
Richard J. Hayes and Richard G. White
London School of Hygiene and Tropical Medicine, London, United Kingdom
To the Editor-Effective preventive interventions against heterosexual HIV transmission rely on an accurate understanding of HIV transmission dynamics. A key question is how the rate of transmission of HIV per coital act varies during the course of infection. Few empirical data have been published on this issue, and we are dependent mostly on the careful longitudinal studies performed in the Rakai district of Uganda.
In a recent issue of the Journal of Infectious Diseases, Wawer et al. [1] presented an elegant analysis of retrospective data on the Rakai study cohort. From 235 monogamous HIV-negative partners of HIV-infected index cases, they obtained estimates of the transmission rate per coital act according to stage of HIV infection. The highest transmission rate, 8/1000 coital acts, was observed during the first 5 months after seroconversion, falling to 1/1000 coital acts during the long period of asymptomatic infection and increasing thereafter to 4/1000 coital acts during late-stage infection, from 6 to 15 months before the death of the index case. No estimates are presented for the last 5 months before death, although the authors suggest that coital frequency is likely to decrease during late-stage infection.
On the basis of these important data, commentators [2] pointed to the importance of early-stage infection for HIV transmission, stating that "nearly one-half of the HIV transmission events observed could be ascribed to a sex partner with newly acquired HIV infection" (p. 1391) and calling for interventions targeted at acute HIV infection. For 3 main reasons, we caution about overestimating the proportion of transmission events in sub-Saharan Africa that are attributable to acute infection.
First, the implications of these data for viral spread depend on the sexual behavior of HIV-infected individuals. The white bars in figure 1 show the proportion of HIV transmission events, based on the Rakai estimates, that occur during each stage of infection, under the assumption that the HIV seroconverter has only 1 subsequent HIV-negative sex partner. As many as 41% of transmission events would occur during the first 5 months after seroconversion; a further 44% would occur during the subsequent asymptomatic period, from 6 months to 8 years after seroconversion; and 14% would occur during late-stage infection, from 8 years after seroconversion until death (assumed to occur after 10 years [3]). However, many HIV-infected individuals will have additional sex partners during the course of their infection, and this significantly alters the pattern. The black bars in figure 1 show the other extreme, in which the seroconverter has the same coital frequency but all sexual contacts are susceptible. This might approximate the situation among sex workers in a relatively low-prevalence setting. In this case, only 23% of secondary infections are attributable to early-stage infection, 46% to the asymptomatic period, and 30% to late-stage infection.
Figure 1. Proportion of HIV transmission events according to time from infection, if the seroconverter is assumed to have only 1 subsequent sex partner (white bars) or if all subsequent sexual contacts are with susceptible individuals (black bars). Assumptions include that the time from infection to death is 10 years and that transmission does not occur during the 5 months before death.
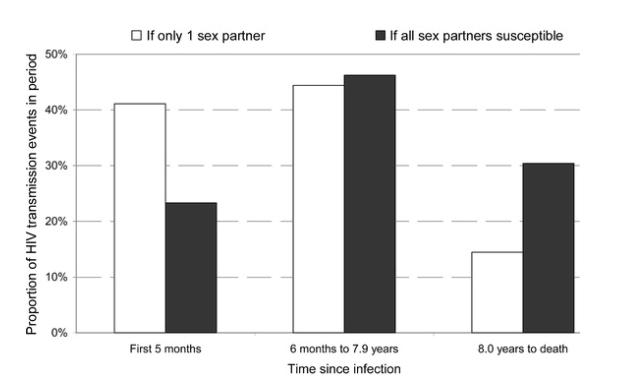
Second, the Rakai studies have demonstrated the importance of biological cofactors such as genital ulcer disease (GUD), which substantially enhance the risk of transmission per coital act. Detailed analyses have shown that prevalences of sexually transmitted infections (STIs) in Ugandan cohorts were relatively low during the period of the studies [4]. In settings with higher STI rates, particularly where GUD is common, the low HIV transmission rate during the asymptomatic period is likely to increase significantly, as is illustrated in figure 1B of the editorial commentary by Cohen and Pilcher [2], along with the proportion of HIV transmission events.
Third, the relative importance of different stages of infection will evolve during the course of an HIV epidemic. In a concentrated or early epidemic, most infections may occur among high-risk groups with many sex partners, so that the black bars in figure 1 may be closer to the truth, although, initially, few HIV-infected subjects will have reached late-stage infection. Later in a generalized epidemic, more infections may occur among individuals with few partners, and the pattern will change to more closely resemble that shown by the white bars in figure 1. In a contracting epidemic, as is currently seen in Uganda, the number of incident infections is small in comparison with the number of prevalent infections, so that, even if most couples are monogamous, most new infections will be attributable to the later stages of infection. In the Rakai study [1], for example, only 10 (15%) of 68 seroconversions were attributable to transmission by acutely infected individuals (during the first 5 months of infection), and not one-half of them, as is suggested in the commentary.
In summary, HIV-negative partners of acutely infected individuals are clearly at very high risk, but the population-level effects of interventions targeted at acutely infected individuals will depend on the sexual behavior of HIV-infected individuals, the stage and extent of the HIV epidemic, and the prevalence of STIs and other biological cofactors.
Amplified HIV Transmission and New Approaches to HIV Prevention
Editorial
Journal of Infectious Diseases May 1, 2005
Myron S. Cohen and Christopher D. Pilcher
Departments of Medicine, Microbiology, and Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill
HIV has infected 40,000,000 people, killed >6,000,000 people, and devastated whole sectors of societies [1]. HIV infection is rightly considered by the United Nations to be a major threat to global security and economy [2]. Prevention of the spread of HIV demands precise knowledge of the biology and epidemiology of transmission [3], a challenge that even now has not been completely met. Although several studies of HIV epidemiology (reviewed in [4]) have described heterosexual transmission as occurring in 1/1000 coital acts, this number seems far too low to explain the magnitude of the HIV pandemic.
In this issue of the Journal of Infectious Diseases, Wawer et al. [5] provide a new and important understanding of the transmission of HIV. Between 1994 and 1999, the investigators enrolled 15,127 subjects from 56 villages in the Rakai District of Uganda in a trial designed to determine whether intermittent mass therapy directed against sexually transmitted infections (STIs) could reduce the spread of HIV. Although this intervention did not reduce the incidence of HIV infection [6], meticulous and extensive collection of demographic, clinical, and historical data on the participants has led to a steady and compelling stream of analysis [79].
In their earlier work [8], the investigators demonstrated that the risk of HIV transmission correlated strongly with blood viral burden and the incidence of genital ulcer disease [9]. In their current work, Wawer et al. [5] make another critically important observation: nearly one-half of the HIV transmission events observed could be ascribed to a sex partner with newly acquired HIV infection.
Although several "couples studies" of HIV transmission [10, 11] have been undertaken in the past, such studies depend on the identification of an index patient with established HIV infection. However, the Rakai investigators retrospectively "constructed" couples from the entire study population based on the subjects' histories and (more recently) viral genetics [12]. This approach allowed them to detect the transmission of HIV within couples even when both partners were HIV seronegative at the beginning of the study.
The authors identified 235 couples for study. On the basis of the number of coital acts reported by both partners, they estimated the probability of HIV transmission from a subject with early infection (an average of 2.5 months after seroconversion) as 8.2 cases/1000 coital acts, with established infection as 715 cases/10,000 coital acts, and with advanced (unrestrained and untreated) infection as 2.8 cases/1000 coital acts. Furthermore, the risk ascribed to patients with early infection is likely an underestimate, given that the data collected did not allow for the detection of subjects with (preseroconversion) acute HIV infection, who are likely to have the highest blood and genital-tract HIV burden [13] and who may have STIs as well [9, 14, 15]. The authors estimate that the risk for HIV transmission from patients with acute infection might be as high as 1 case/50 coital actsgreater than the transmission risk associated with deep needlestick injuries [16].
These results strongly support earlier modeling predictions. Using blood and semen samples harvested from patients at different stages of disease, Chakraborty et al. [4] constructed a probabilistic model of HIV transmission. According to this model, the very high viral burden in semen that has been demonstrated in patients with acute HIV infection should result in an 810-fold increase in the risk of male-to-female transmission [13] (figure 1A). Coinfection with "classic" STI pathogens [17, 18] and high-risk behavior in acutely infected patients [19] would also amplify transmission in sexual networks [20] (figure 1B). As early as 1994, Koopman et al. [20] and Jacquez et al. [21] used population modeling to argue that the spread of HIV from patients with early, transient hyperinfectiousness could contribute disproportionately to the epidemic.
Figure 1. Prediction of the efficiency of HIV transmission according to HIV burden in the genital tract. A, Probability of male-to-female HIV transmission per coital act, as a function of HIV disease stage in the index case. Transmission probabilities are shown for each stage of disease [35, 13]. Yellow, Expected distribution of viral burden in semen among men over time; red, theoretical effect of a biological intervention designed to reduce viral excretion; dashed line, a potential threshold for HIV transmission. B, Determinants of high HIV transmission probability: acute infection, sexually transmitted infection (STI), and AIDS.
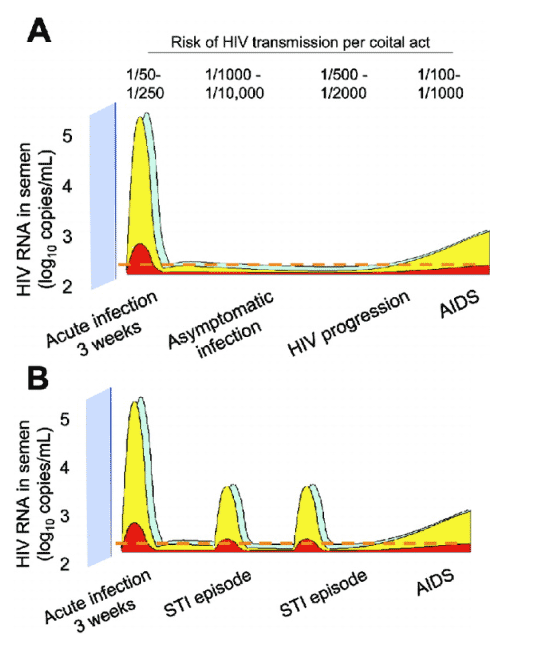
When investigators have searched for people with acute HIV infection, they have been found. Rosenberg et al. [22] reported that 1.0% of patients with negative tests for Epstein-Barr virus infectious mononucleosis had serological results consistent with acute HIV infection. Pincus et al. [23] found that 1.0% of patients with "any viral symptoms" in a Boston urgent-care center had unsuspected acute HIV infection. In a Malawi STI clinic, 2.8% of all male clients with acute STIs had acute HIV infection [18]. However, acute retroviral symptoms occur in only one-half of patients, and the signs and symptoms are nonspecific [24]. Furthermore, the clinical diagnosis of acute HIV has relied on tests (HIV p24 antigen or nucleic-acid amplification tests) that add significantly to the costs of testing and have been dogged by concerns with specificity. Recent innovations in high-throughput group testing for HIV RNA in antibody-negative specimens allow more efficient identification of people with acute infection, regardless of clinical presentation [25, 26]. Not surprisingly, such patients are most often detected in STI clinics [27], which supports the importance of cotransmission of HIV and STIs.
If patients with acute HIV infections can be more readily detected, the opportunities for novel care and prevention are considerable. The results of clinical trials have suggested that very early antiretroviral therapy and/or immune-based therapy may benefit the acutely HIV-infected patient [2830]. In addition, aggressive attempts to reduce the size of the latent HIV pool in these patients are likely to be forthcoming [31].
Prevention of HIV transmission is of paramount importance. Historically, HIV prevention efforts have focused on HIV-uninfected subjects, whereas prevention directed at infected subjects has only gained attention very recently [32, 33]. To respond adequately to the threat of amplified HIV transmission, HIV prevention strategies must use complementary diagnostic, behavioral, and biological tools. Unique partner notification, counseling and referral services [34], and novel biological interventions should be developed specifically for people with acute HIV infection. Wawer et al. [5] have confirmed the remarkable threat of HIV transmission posed by people with newly acquired HIV infection. The challenge now is to waste no time in finding the most creative strategies to incorporate these results into global HIV prevention efforts.
Viral Load and Heterosexual Transmission of HIV Type 1
NEJM March 30, 2000, Vol 342:921-929
Thomas C. Quinn, M.D., Maria J. Wawer, M.D., Nelson Sewankambo, M.B., David Serwadda, M.B., Chuanjun Li, M.D., Fred Wabwire-Mangen, Ph.D., Mary O. Meehan, B.S., Thomas Lutalo, M.A., Ronald H. Gray, M.D., for The Rakai Project Study Group
ABSTRACT
Background and Methods We examined the influence of viral load in relation to other risk factors for the heterosexual transmission of human immunodeficiency virus type 1 (HIV-1). In a community-based study of 15,127 persons in a rural district of Uganda, we identified 415 couples in which one partner was HIV-1-positive and one was initially HIV-1-negative and followed them prospectively for up to 30 months. The incidence of HIV-1 infection per 100 person-years among the initially seronegative partners was examined in relation to behavioral and biologic variables.
Results The male partner was HIV-1-positive in 228 couples, and the female partner was HIV-1-positive in 187 couples. Ninety of the 415 initially HIV-1-negative partners seroconverted (incidence, 11.8 per 100 person-years). The rate of male-to-female transmission was not significantly different from the rate of female-to-male transmission (12.0 per 100 person-years vs. 11.6 per 100 person-years). The incidence of seroconversion was highest among the partners who were 15 to 19 years of age (15.3 per 100 person-years). The incidence was 16.7 per 100 person-years among 137 uncircumcised male partners, whereas there were no seroconversions among the 50 circumcised male partners (P<0.001). The mean serum HIV-1 RNA level was significantly higher among HIV-1-positive subjects whose partners seroconverted than among those whose partners did not seroconvert (90,254 copies per milliliter vs. 38,029 copies per milliliter, P=0.01). There were no instances of transmission among the 51 subjects with serum HIV-1 RNA levels of less than 1500 copies per milliliter; there was a significant dose-response relation of increased transmission with increasing viral load. In multivariate analyses of log-transformed HIV-1 RNA levels, each log increment in the viral load was associated with a rate ratio of 2.45 for seroconversion (95 percent confidence interval, 1.85 to 3.26).
Conclusions The viral load is the chief predictor of the risk of heterosexual transmission of HIV-1, and transmission is rare among persons with levels of less than 1500 copies of HIV-1 RNA per milliliter.
HIV-1 RNA Levels and the Risk of Transmission
Of the 415 seropositive partners, 364 (88 percent) had detectable serum levels of HIV-1 RNA. The mean serum level of HIV-1 RNA among the 228 HIV-1-positive men was 59,591 copies per milliliter (median, 15,649) and was significantly higher than the mean level of 36,875 copies per milliliter among the 187 HIV-1-positive women (median, 9655; P=0.03). When the log-transformed values were used, the mean (±SD) value was 4.11±0.86 log copies of HIV-1 RNA among the men and 3.90±0.83 log copies of HIV-1 RNA among the women (P=0.008) (Table 3). Among couples in which the initially HIV-1-negative partner seroconverted, the mean serum HIV-1 RNA level of the HIV-1-positive partner was significantly higher than that of the HIV-1-positive partner in couples in which the HIV-1-negative partner remained seronegative (mean, 90,254 copies per milliliter vs. 38,029 copies per milliliter; P=0.01). When these two subgroups were analyzed according to sex, the log-transformed values were significantly higher among male and female subjects whose partners seroconverted than among male and female subjects whose partners did not seroconvert (P=0.001) (Table 3).
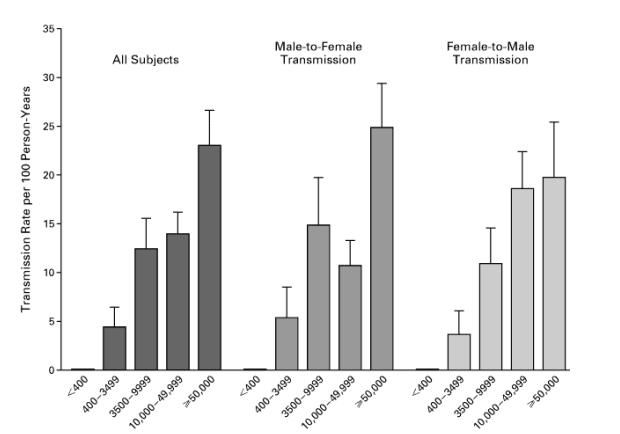
There was a significant dose-response effect with respect to both male-to-female transmission and female-to-male transmission (P<0.001) (Figure 1). The rate of transmission was zero among the 51 couples in which the HIV-1-positive partner had undetectable serum levels of HIV-1 RNA or less than 1500 copies per milliliter. Among HIV-1-positive partners with serum HIV-1 RNA levels of less than 3500 copies per milliliter, the rate of transmission was 2.2 per 100 person-years, and the rates progressively increased with increasing viral loads, to a maximum of 23.0 per 100 person-years at a level of 50,000 or more copies per milliliter. It is noteworthy that among the 90 instances of transmission, 5.6 percent occurred among couples in which the HIV-1-positive partner had serum HIV-1 RNA levels of 400 to 3499 copies per milliliter, 17.7 percent among couples in which the seropositive partner had levels of 3500 to 9999 copies per milliliter, 40.0 percent among couples in which the seropositive partner had levels of 10,000 to 49,999 copies per milliliter, and 36.7 percent among couples in which the seropositive partner had levels of 50,000 or more copies per milliliter. There was no significant difference between male-to-female and female-to-male transmission rates after the results were adjusted for viral load (P=0.76), and there were no consistent differences between male-to-female or female-to-male transmission rates within strata of viral load (Figure 1).
Figure 1. Mean (+SE) Rate of Heterosexual Transmission of HIV-1 among 415 Couples, According to the Sex and the Serum HIV-1 RNA Level of the HIV-1-Positive Partner.
At base line, among the 415 couples, 228 male partners and 187 female partners were HIV-1-positive. The limit of detection of the assay was 400 HIV-1 RNA copies per milliliter. For partners with fewer than 400 HIV-1 RNA copies per milliliter, there were zero transmissions.
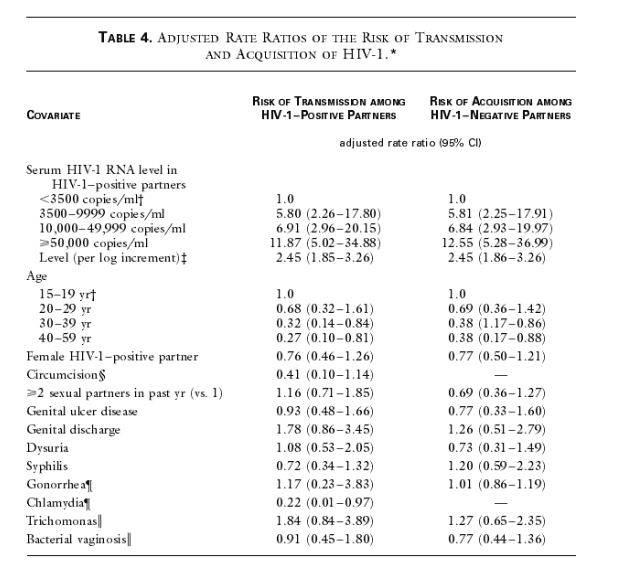
Results of Multivariate Logistic-Regression Analysis
We constructed several Poisson regression models, the results of which are summarized in Table 4. Viral load was the variable most strongly predictive of the risk of transmission. When viral load was measured as a categorical variable, with HIV-1-positive partners with serum HIV-1 RNA levels of less than 3500 copies per milliliter as the reference group, the rate ratio of the risk of transmission increased from 5.80 (95 percent confidence interval, 2.26 to 17.80) for HIV-1-positive subjects with HIV-1 RNA levels of 3500 to 9999 copies per milliliter to 11.87 (95 percent confidence interval, 5.02 to 34.88) for seropositive subjects with 50,000 or more copies per milliliter. When viral load was measured as a continuous variable, the rate ratio for the risk of transmission associated with each log increment in viral load was 2.45 (95 percent confidence interval, 1.85 to 3.26).
As compared with the risk of transmission among HIV-1-positive partners who were 15 to 19 years of age, the risk of transmission decreased with older age, after adjustment for viral load, and this decrease was significant for those who were 30 to 39 years of age (rate ratio, 0.32) and those who were 40 to 59 years of age (rate ratio, 0.27). The interaction between age and log-transformed viral load was not statistically significant (P=0.06). The risk of transmission was lower among circumcised male subjects than among uncircumcised male subjects, but this difference was not significant (rate ratio, 0.41; 95 percent confidence interval, 0.10 to 1.14).
The risk of infection increased as the HIV-1-infected partner's viral load increased and decreased with age among HIV-1-negative partners. The risk of infection was zero among the 50 HIV-1-negative circumcised male subjects. A history of multiple sexual partners, symptoms of sexually transmitted diseases, or the laboratory diagnosis of sexually transmitted diseases had no significant effect on the risk of HIV-1 infection.
Discussion
Prospective studies of HIV-1-discordant couples provide important information on the efficiency of transmission and the biologic and behavioral variables that influence the infectiousness of and susceptibility to HIV-1. Our study of heterosexual transmission among sexual partners was a community-based study in which all consenting couples, whether discordant for HIV-1 or not, were prospectively followed to evaluate the risk of transmission in relation to viral load and other characteristics. Our study sample is representative of the general population in this rural area of Uganda.
All participants were asked whether they wanted to know the results of their HIV-1 tests, all were offered counseling after testing and free condoms in the privacy of their own homes, and all were told about safe-sex practices. Couples counseling was also offered to the entire community, and all subjects were strongly encouraged to share the results of testing with their partners. Although the rate of condom use remained low in the entire study population, as has been the case in other studies in Uganda,28 we did observe an increase in current condom use over the four-year study, from 4.4 percent to 7.4 percent as reported by women and from 9.9 percent to 16.9 percent as reported by men; these values represent some of the highest rates of use in rural sub-Saharan Africa. However, with this rate of condom use, HIV-1 was transmitted to 90 of the 415 initially HIV-1-negative partners, for an overall incidence of 11.8 per 100 person-years. This was significantly higher than the incidence of 1.0 per 100 person-years reported among couples in which both members were initially seronegative.21
The major finding of this study was the strong association between increasing serum HIV-1 RNA levels and an increasing risk of heterosexual transmission of HIV-1. In a finding similar to those of studies that found that the risk of perinatal HIV-1 infection is associated with the maternal viral load,24,29,30,31,32 we found a dose-response effect: the rate of transmission increased from 2.2 per 100 person-years to 23.0 per 100 person-years as the serum HIV-1 RNA level increased from less than 3500 copies per milliliter to 50,000 or more copies per milliliter (adjusted rate ratio, 11.87). In multivariate analyses, the serum HIV-1 RNA level was the main predictor of the risk of transmission (Table 4). Each log increase in viral load was associated with an increase by a factor of 2.45 in the risk of transmission. There were no instances of transmissions by seropositive subjects with undetectable viral loads or with serum HIV-1 RNA levels of less than 1500 copies per milliliter. This finding raises the possibility that reductions in viral load brought about by the use of antiretroviral drugs could potentially reduce the rate of transmission in this population. Such reductions in transmission have been documented in studies of perinatal transmission,30,32,33 but not in studies of sexual transmission. Further studies measuring the effects of antiretroviral drugs on sexual transmission are urgently needed.
Several studies have shown a good correlation between peripheral-blood viral load and viral load in seminal plasma34 and cervical secretions,35,36 and viral loads in genital secretions appear to fall in concert with the declines in peripheral-blood viral load after combination therapy.20,34,37 However, the rate of transmission of HIV-1 was not assessed in these studies, and despite reductions in peripheral-blood and seminal plasma viral load, integrated viral DNA is still present in seminal cells, and virus can be recovered in vitro.38,39,40 However, it is apparent from our results that the rate of transmission is markedly reduced among persons with very low serum viral loads.
In multivariate analyses, we did not find a significant association between the risk of HIV-1 transmission and the presence of sexually transmitted diseases or symptoms of sexually transmitted diseases in HIV-1-positive partners, or between an increased susceptibility to infection and sexually transmitted diseases among HIV-1-negative partners. However, genital discharge and dysuria in the seropositive partner were significant in the unadjusted analysis. This last finding, even though not significant in the multivariate analysis, is compatible with findings from other studies in which persons with a genital discharge had increased HIV-1 RNA levels in genital secretions.41,42
In analyses of the risk of transmission according to male or female sex, we found no significant difference in incidence between female-to-male transmission and male-to-female transmission. The rate in each group was about 12 per 100 person-years. For each category of viral load, the rates of transmission were similar in both sexes, and these results reflect the nearly equal distribution of HIV-1 infection between men and women in this community and in most other parts of Africa.1,43 The transmission rates reported here reflect a combination of the probability of transmission per sexual act, the frequency of sexual contact, viral shedding in the genital tract as influenced by the presence of concurrent genital tract infections, and other variables.
Despite similarities in transmission rates between the sexes at each level of viral load, seropositive female subjects did have significantly lower log-transformed mean viral loads than male subjects, and this sex-specific difference was greatest among the subjects who transmitted the virus to their partners (mean log-transformed viral load, 4.30 among seropositive female subjects and 4.62 among seropositive male subjects; P=0.015). These data are consistent with recent reports that female subjects have lower viral loads than male subjects matched with them for age and CD4 count, despite the fact that they had similar rates of progression and similar decreases in the CD4 count.44,45 The mechanisms for these sex-based differences in viral load are unclear.
An additional finding in our study was that circumcision was protective against HIV-1 infection, with no infections occurring among 50 circumcised HIV-1-negative male subjects, as compared with 40 infections among 137 HIV-1-negative uncircumcised male subjects. This finding suggests that male circumcision may reduce the risk of acquisition at all HIV-1 RNA levels. Studies among truck drivers, persons attending sexually transmitted disease clinics, and prostitutes and their clients in Africa have shown that the absence of circumcision among men increases their risk of heterosexual acquisition of HIV-1,9,11,46 potentially because of an association with an increased frequency of sexually transmitted diseases among uncircumcised men.11 This association between male circumcision and a decreased risk of infection with HIV-1 may partially explain the low frequency of female-to-male transmission in U.S. studies of HIV-1-discordant couples,47 since over 70 percent of men in the United States are circumcised.
Limitations in the interpretation of our data include the fact that the interval between the measurement of the viral load in the index subject and documentation of seroconversion in the partner was 10 months, resulting in some imprecision as to the viral load at the time of transmission. Similarly, the diagnosis of sexually transmitted diseases was established at the visit before and the visit after the end of the interval in which there was a risk of seroconversion, which may have diluted the potential association between sexually transmitted diseases and the risk of transmission of HIV-1. However, data on symptoms of sexually transmitted diseases were available for the entire interval in which there was a risk of seroconversion, and serum viral load was a much stronger predictor of the risk of transmission than was the presence of such symptoms in either partner.
Heterosexual transmission involves a complex interaction between biologic and behavioral factors. Our data suggest that peripheral-blood levels of HIV-1 RNA contribute dramatically to the risk of heterosexual transmission. Serum HIV-1 RNA levels below 1500 copies per milliliter were not associated with transmission, whereas the risk of transmission increased substantially with increasing viral loads. These results suggest that research is urgently needed to develop and evaluate cost-effective methods, such as effective and inexpensive antiretroviral therapy or vaccines, for reducing viral load in HIV-1-infected persons. Such measures, coupled with education about safe-sex practices, condom use, HIV-1 testing and counseling, and control of sexually transmitted diseases, could potentially reduce the infectivity of and susceptibility to HIV-1 and prevent further sexual transmission of the virus.
Supported by grants (R01 AI34826b and R01 AI34826S) from the National Institute of Allergy and Infectious Diseases; by a grant (5P30HD06826) from National Institute of Child Health and Human Development; and by the Rockefeller Foundation and the World Bank Uganda Sexually Transmitted Infections Project. Some drugs and laboratory tests were provided by Pfizer, Abbott Laboratories, Roche Molecular Systems, and Calypte Biomedical.
We are indebted to S. Sempala (Uganda Virus Research Institute, Uganda Ministry of Health) for his support of the study; to Sharon Hillier (University of Pittsburgh) for reviewing the vaginal swabs for bacterial vaginosis; to Patricia Buist for editorial assistance; to Richard Kline, Christopher Urban, and Denise McNairn for performing viral-load assays; to all the study participants in Rakai District, Uganda, for their contributions and support; and to Anthony Fauci, M.D., and Rodney Hoff, M.D., for their helpful suggestions regarding the manuscript.
|
|
| |
| |
|
|
|