| |
Challenges of developing R5 inhibitors in antiretroviral naive HIV-infected patients
|
| |
| |
The Lancet March 4, 2006; 367:711-713
Steven G Deeks a
The success of combination antiretroviral therapy in preventing progression to AIDS is one of the great success stories of modern medicine. Although there remains uncertainty, we can anticipate that most HIV-infected individuals will have a normal life-expectancy, assuming that they are able to access and adhere to a standard combination antiretroviral regimen. Is there still a compelling need for new therapeutic options in treatment-naive individuals? This question is central to recent controversies regarding the development of R5 inhibitors, a newly developed therapeutic class of antiretroviral drugs.
HIV enters CD4+ T cells via an interaction with CD4 and at least one chemokine receptor (CCR5 or CXCR4). Most people with early HIV disease harbour a virus which uses CCR5 (R5 virus); viruses using CXCR4 (X4 virus) are generally detectable only in late-stage disease. Individuals who carry a genetic deletion in the gene for CCR5 are less susceptible to becoming HIV-infected, and progress less rapidly when infected.1 Despite the lack of CCR5, individuals homozygous for this deletion have a normal lifespan. Given these observations, several drug companies invested heavily in drugs that bind to CCR5, thereby preventing R5 variants from entering their target cells (table). Preliminary data indicate that these R5 inhibitors are highly effective in reducing HIV replication in vivo, at least during short-term follow-up.2,3
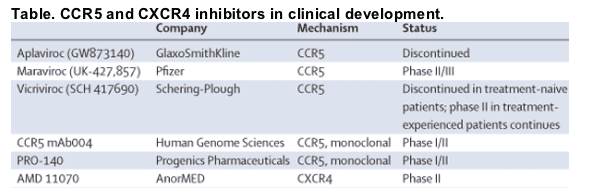
In September, GSK suddenly announced the termination of their phase II/III programme to evaluate the safety of aplaviroc in treatment-naive patients. Clinical investigators were sent an urgent letter requesting that all participants stop randomised drug treatment within 48 h. This decision was initially made because of severe, yet reversible, liver toxicity in at least one patient, and asymptomatic rises in liver enzymes in several other patients. Treatment-experienced patients with multidrug-resistant HIV who were enrolled in aplaviroc gsalvageh studies were initially allowed to continue taking the study drug, but all studies were permanently discontinued on Oct 25, 2005, when it became clear that severe hepatotoxicity had occurred in several patients.4
These events raise several issues relevant to the development of novel antiretroviral drugs. Perhaps the most immediate issue is the mechanism by which aplaviroc caused hepatotoxicity. If the toxicity was due to a direct effect (or an allergic reaction), chemically distinct R5 inhibitors should remain safe. If, however, the toxicity was a result of CCR5 inhibition, then the entire therapeutic approach will need to be reconsidered. Two studies provide some reason for concern: mice who lack CCR5 and who were exposed to hepatotoxins were found to be highly suspectible to the development of immune-mediated fulminant liver failure.5,6 For now, the clinical development of R5 inhibitors continues, with all investigators monitoring liver enzymes closely. Fortunately, hepatotoxicity has not been consistently associated with the other R5 inhibitors, although there was one case of liver failure in an individual co-infected with hepatitis C treated with maraviroc and several other potentially hepatotoxic drugs.
The second issue raised by GSK's trial termination is their initial decision to stop aplaviroc in treatment-naive patients but allow the drug to be continued in the heavily pretreated gsalvageh patients. This decision reflects an emerging split of the epidemic into two distinct populations: those with several highly effective and well-tolerated treatment options, and those without such options because they have highly-drug-resistant HIV. For the motivated treatment-naive individual infected with drug-susceptible HIV, as few as two pills taken once a day will result in durable and perhaps permanent suppression of HIV to undetectable levels.7 The long-term prognosis for these patients is now so favourable that many organisations, including the community-based European AIDS Treatment Group,8 heavily criticised GSK and other drug companies for performing a study that randomises treatment-naive patients to a potentially unsafe drug such as aplaviroc. This criticism undoubtedly contributed to the rapid termination of the phase II aplaviroc programme in treatment-naive patients.
The situation for the treatment-experienced patient is very different. These patients, who make up 10-20% of most clinic-based cohorts, generally began therapy before the availability of combination therapy and out of necessity continue to be exposed to sequential monotherapy.9,10 Because many of these patients are at high risk of clinical progression, the potential benefit of these drugs typically outweighs any potential risks. For this reason, GSK initially chose to continue aplaviroc in the treatment-experienced patients. Similarly, the novel protease inhibitor, tipranavir, was recently granted accelerated approval in the USA and Europe despite clear evidence of hepatotoxicity. The label clearly states that its use should be limited to the treatment-experienced patient who has few other treatment options.
Any discussion regarding the pros and cons of developing a new drug for the treatment-naive patient needs to consider the effectiveness of current therapeutic options. In controlled clinical studies, current regimens routinely suppress HIV RNA levels to undetectable levels for long periods, with overall success rates of over 80% reported with conservative intent-to-treat analytic techniques (many gfailuresh in these studies are due to reasons other than virological rebound).11,12 These virological success rates are so high that any new regimen must achieve and maintain undetectable viral loads in the vast majority of patients. This efficacy hurdle led to a recent decision to discontinue a phase II study of vicriviroc (Schering-Plough) in treatment-naive patients. As recently presented at the 13th Conference on Retroviruses and Opportunistic Infections, more patients receiving zidovudine/lamivudine plus vicriviroc experienced virological rebound than those receiving zidovudine/lamivudine plus efavirenz.12 A poor virological response rate also led to discontinuation of the low-dose maraviroc arm in an ongoing phase III study in treatment-naive patients. Importantly, the development of these drugs in the treatment-experienced salvage populations has not been altered. As there are no standard regimens for such patients, maraviroc and vicriviroc are being compared with placebo in these studies. The barrier to showing effectiveness is therefore much lower than that used in treatment-naive patients.
These considerations suggest that drug development should be preferentially limited to treatment-experienced patients, at least until safety and related issues are clarified. But limiting the use of R5 inhibitors to the treatment-experienced patient raises another important concern specific to this drug class. Although most individuals with early-stage HIV infection harbour viruses that require CCR5 for cell entry, 50% or more patients with advanced disease harbour viruses that use CXCR4.1 These viruses are naturally resistant to R5 inhibitors. Recent data suggest that some patients with R5 virus also harbour X4 virus at a low level that is not detectable with current assays.13 In these patients, X4 virus is likely to emerge under the selective pressure of these drugs.3,14 Because patients with X4 virus seem to progress more rapidly than those with R5 virus, the use of these drugs may accelerate disease progression, even if liver toxicity does not occur.15
The continuing saga of how best to study R5 inhibitors illustrates an important dilemma facing all interested parties. Although it is generally easy to study new drugs in the treatment-naive population of patients, such studies may no longer be easy to justify. By contrast, the unmet medical need is so great for the treatment-experienced population that the potential benefit often outweighs the risk. Whether companies will continue to invest heavily in drugs for this population of patients remains unclear, particularly as the number of such patients remains low and the duration of treatment with any new drug will probably be relatively short. Our success in managing the treatment-naive population may eventually limit our ability to manage the treatment-experienced population.
I have received honorarium from GSK, provided informal consultations on the development of aplaviroc, and am a member of a Data and Safety Monitoring Board overseeing the development of an experimental R5 inhibitor.
|
|
| |
| |
|
|
|