| |
Neutropenia in HIV+ Women; survival.
Data From the Women's Interagency HIV Study
|
| |
| |
Alexandra M. Levine, MD; Roksana Karim, MBBS, MS; Wendy Mack, PhD; D. Jay Gravink, MA; Katherine Anastos, MD; Mary Young, MD; Mardge Cohen, MD; Meg Newman, MD; Michael Augenbraun, MD; Stephen Gange, PhD; D. Heather Watts, MD
Arch Intern Med. Feb 27, 2006;166:405-410.
ABSTRACT
Background: Neutropenia is well described in individuals infected with human immunodeficiency virus (HIV) and occurs in approximately 10% to 50% of cases. Neither the effect of highly active antiretroviral therapy (HAART) on neutrophil counts nor the significance of neutropenia in terms of survival has previously been evaluated.
Methods: The prevalence of neutropenia among 1729 HIV-infected women, followed up as part of the Women's Interagency HIV Study, was evaluated. The CD4 lymphocyte counts, HIV-1 RNA levels, and complete blood cell counts, including absolute neutrophil counts, were obtained at 6-month intervals.
Results:
Neutropenia was common among HIV-infected women; at baseline, 44% had neutrophil counts less than 2000/mL, whereas 7% had counts less than 1000/mL. During 7.5 years of follow-up, neutrophil counts less than 2000/mL occurred on at least 1 occasion in 79%, whereas absolute neutrophil counts less than 1000/mL were documented in 31%.
Worsening HIV disease parameters, such as lower CD4 cell counts (P<.001) and higher HIV-1 RNA levels (P<.001), were associated with development of neutropenia. Resolution of neutropenia was associated with higher CD4 cell counts (P<.001) and use of HAART (P=.007). We found that HAART, without zidovudine, was associated with protection against development of neutropenia. On multivariate analysis, neutropenia was not found to be associated with decreased survival among HIV-infected women.
Conclusions: Worsening HIV disease parameters are associated with neutropenia in HIV-infected women. Treatment with HAART, without zidovudine in the regimen, protects against development of neutropenia, whereas HAART use and higher CD4 cell counts are associated with resolution of neutropenia. Neutropenia is not associated with decreased survival in HIV-infected women.
SURVIVAL ANALYSIS
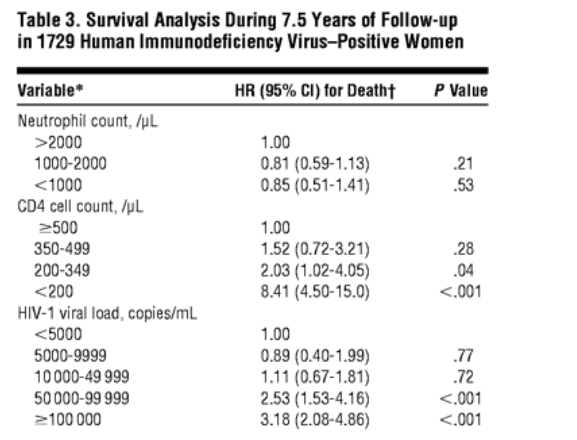
On multivariate Cox proportional hazards analysis, neutropenia was not statistically significantly associated with survival in HIV-positive women (Table 3). As expected, low CD4 lymphocyte count and high HIV-RNA viral load were significant predictors of survival in these women. Compared with women with CD4 lymphocyte counts greater than or equal to 500/mL, women who had CD4 cell counts between 200 and 349/mL were 2 times and those who had CD4 cell counts less than 200/mL were 8 times more likely to die (P = .04 and P<.001, respectively). Women with HIV viral loads between 50 000 and 99 999 copies/mL had 2.5 times greater risk of death, and women with 100 000 copies/mL or greater had a 3 times greater risk of dying, than women who had HIV-RNA viral loads of less than 5000 copies/mL (P<.001). On multivariate analysis, including age, race, CD4 cell counts, and HIV-RNA level, neither total number of visits with neutropenia nor recurrence of neutropenia in 2 or more successive visits (ie, persistent neutropenia) was associated with survival (data not shown).
INTRODUCTION
Infection with human immunodeficiency virus (HIV) is associated with profound effects on hematopoiesis, with anemia, thrombocytopenia, and neutropenia all well described in infected individuals.1-2 Although neutropenia has been reported in approximately 10% to 50% of these patients, relatively little has been studied in terms of the correlates of neutropenia, its significance on overall survival, or the effects of highly active antiretroviral therapy (HAART) in either preventing or correcting the neutropenia associated with HIV.
The Women's Interagency HIV Study (WIHS) is an ongoing evaluation of the course of HIV infection among women. Study participants are evaluated every 6 months, with information obtained by structured interview regarding use of HIV-related medications, intercurrent illness, and other issues, and a comprehensive laboratory evaluation is also performed. Data regarding white blood cell and neutrophil counts and HIV-related laboratory evaluations have been collected.
We analyzed the neutrophil counts in 1729 HIV-infected women, collected every 6 months during a follow-up period of 7.5 years, to ascertain the relationship between neutropenia and the extent and severity of HIV infection, the role of HAART on development or resolution of neutropenia, and the significance of neutropenia in terms of the overall survival of these individuals.
STUDY POPULATION
Details of the WIHS have been described previously.3 In short, the WIHS is a multicenter, prospective study designed to examine the characteristics and course of HIV infection in US females. A total of 2059 HIV-infected and 569 matched controls were enrolled in the study between October 1994 and November 1995 at 6 different sites in the United States (Los Angeles, Calif; northern California; Washington, DC; Bronx and Manhattan, NY; Brooklyn, NY; and Chicago, Ill). Informed written consent was obtained from the study participants, and the study was approved by the institutional review boards at each of the 6 sites.
DEFINITION OF HAART
At each visit, participants were asked about their current ART use and ART use in the past 6 months. The definition of HAART regimens was guided by the 2000 versions of the US Department of Health and Human Services4 and the International AIDS Society-USA Panel antiretroviral guidelines.5 Women were considered to be receiving HAART if they were taking (1) 2 or more nucleoside reverse-transcriptase inhibitors (NRTIs) with at least 1 protease inhibitor (PI) or nonnucleoside reverse-transcriptase inhibitor (NNRTI); (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI; (3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs; or (4) an abacavir sulfate-containing regimen of 3 or more NRTIs in the absence of both PIs and NNRTIs. Monotherapy included either 1 NRTI (95%) or only PIs (4%) or only NNRTIs (1%). All other regimens were defined as combination therapy.
COMMENT
This analysis included 7.5-year follow-up data. The maximum possible number of visits was 16, which occurred between July 1, 2002, and September 30, 2002.
This longitudinal study of a large number of HIV-infected women has demonstrated that neutropenia is clearly associated with more advanced characteristics of HIV disease, including low CD4 cell counts and high HIV-RNA levels in plasma. Moreover, the study is the first to demonstrate that HAART may prevent the development of neutropenia in women with normal neutrophil counts. Notably, we have also shown that HAART is statistically associated with resolution of neutropenia in HIV-infected women with neutrophil counts less than 2000/mL. Of interest, although ART was associated with prevention of neutropenia, inclusion of zidovudine in the antiretroviral regimen seemed to ameliorate this ability. These findings would be fully consistent with the myelosuppression known to occur with zidovudine.8-9 The use of myelosuppressive medications is the most common cause of cytopenias, including neutropenia,10-11 in the setting of HIV infection, and prior studies have also shown that HIV-infected patients with neutropenia are significantly more likely to be receiving zidovudine or trimethoprim-sulfamethoxazole than nonneutropenic patients.12 In a study11 of 87 consecutive HIV-infected patients with ANCs less than 2000/mL, only 3 individuals were not receiving drugs associated with neutropenia, and 66% were receiving 3 or more potentially myelosuppressive medications. The direct toxic effect of zidovudine on bone marrow function may dampen the ability of HAART to overcome HIV-induced neutropenia through its effects in reducing the viral load. Because of these data, it would seem prudent to avoid zidovudine-containing HAART regimens in patients with severe anemia (hemoglobin level <10 g/dL).
Other factors may also contribute to the neutropenia seen in the setting of HIV. Progenitor cell growth is clearly abnormal in HIV-infected individuals, affecting red cells and platelets in addition to neutrophils, with significant reduction in the number of granulocyte-macrophage colony-forming units demonstrated in vitro.13-16 This finding would be consistent with the fact that both anemia and thrombocytopenia were risk factors for neutropenia among our study participants. The presence of soluble inhibitory substances, produced by HIV-infected cells, has been shown to suppress neutrophil production in vitro.16 Decreased serum levels of granulocyte colony-stimulating factor have been described in HIV-seropositive patients with neutropenia, indicating that a relative deficiency of this specific hematopoietic growth factor may contribute to persistent neutropenia in these individuals.17-18 Autoimmune phenomena have also been described.19 Additionally, bone marrow infiltration by infectious organisms or by malignant cells may be operative in the development of neutropenia in HIV-infected individuals.
As discussed, many of the possible causes of neutropenia in the setting of HIV involve either direct or indirect effects of the virus itself against hematopoietic progenitors or growth factors. The ability of HAART to prevent the neutropenia of HIV infection or to correct this cytopenia, as demonstrated in the present study, would thus appear biologically plausible and consistent with the finding that HAART may normalize the function of hematopoietic progenitors.20 Huang et al21 have shown a statistically significant increase in peripheral blood granulocytes after the use of effective ART in a group of 66 HIV-infected patients, 1 of whom was female. However, in contrast to our own results, Huang and colleagues found no relationship between neutropenia and CD4 cell count or HIV-1 viral load. This discrepancy may be related to the fact that the study by Huang and colleagues involved only 66 individuals followed up for 18 months, whereas the current study involved approximately 1700 individuals followed up for as long as 7.5 years.
In our study, neutropenia (defined as an ANC of either <1000/mL or <2000/mL) was not an independent risk factor for survival, a finding not previously reported. Severe neutropenia per se is clearly a risk factor for bacterial infection, with ANCs less than 1500/mL statistically associated with an increased risk of serious bacterial infection among patients with acute leukemia, with increasing risk as the neutrophil count falls below this threshold.22Other factors associated with infection include the duration of neutropenia22 and, in the setting of HIV infection, the level of CD4 cells.11 Of interest, although neutropenia is relatively common among HIV-infected persons and despite the fact that neutrophil function is impaired in the setting of HIV,23-24 serious bacterial infections is relatively uncommon in this setting. Moore et al12 noted that 7.2% of 1638 HIV-infected individuals had neutrophil counts less than 1000/mL; there was no statistically increased risk of any individual bacterial infection among these individuals. However, when all infections were combined, the adjusted relative risk of bacterial infection was approximately 2 times higher than that seen in the nonneutropenic patients (P = .05).12 In a group of 62 HIV-infected patients with ANCs less than 1000/mL, studied by Meynard et al,10 24% developed infectious complications. Factors associated with infection included diagnosis of lymphoma, use of chemotherapy, neutropenia in the previous 3 months, use of central venous catheters, and a lower ANC. Thus, although neutropenia is clearly independently associated with development of infection,12 the risk of such infection is still relatively low and less than that expected in other patient populations.22 The reasons for this discrepancy are unknown but may relate to the relatively short duration of neutropenia among HIV-infected patients, lasting a median of 13 days in the study by Moore et al,11 or to the relatively moderate degree of neutropenia seen in this setting.10-11 Nonetheless, despite low neutrophil counts, most HIV-infected patients do not develop serious bacterial infections, consistent with the fact that neutropenia was not associated with decreased survival in our study. These data would suggest that granulocyte colony-stimulating factors be used conservatively in patients with mild or moderate HIV-related neutropenia.
Our study was not able to elucidate the rate of bacterial infections that occurred during the 7.5 years of follow-up in study participants or the specific types of infections that occurred. Furthermore, we do not have full information regarding the complete medication history of participants. Nonetheless, this large, prospective study was powered to enable us to elucidate those factors associated with neutropenia among HIV-infected women and to define the factors associated with prevention of neutropenia or resolution of preexisting neutropenia.
In summary, neutropenia is common in HIV-infected women, occurring in 44% at baseline and in 79% during 7.5 years of follow-up. Neutropenia is associated with worsening HIV disease parameters and may be prevented or reversed by use of effective ART, which does not include zidovudine. Although bacterial infections may be increased among HIV-infected patients with neutropenia, this study has shown that overall survival is not adversely affected. Future study of the mechanisms that spare most of these individuals from developing severe bacterial infections would be of great interest.
RESULTS
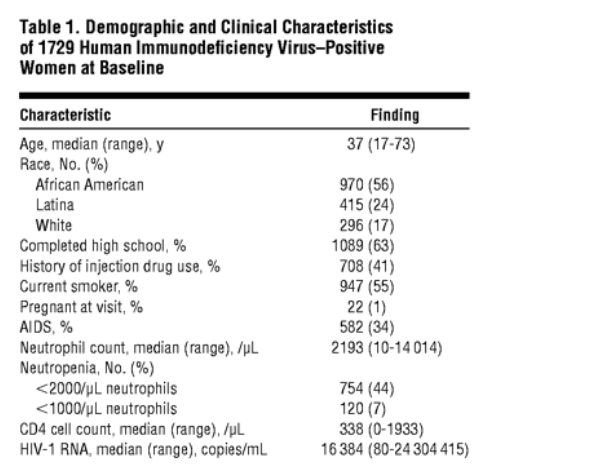
A total of 1729 HIV-positive women had neutrophil counts available for the analysis. At baseline, the median age of the participants was 37 yearas (age range, 17-73 years; Table 1). Most women (56%) were African American,had completed high school (63%), and were current smokers (55%). The median neutrophil count was 2193/mL (range, 10-14 014/mL). Of these 1729 women, 754 (44%) were neutropenic at baseline, when neutropenia was defined as a neutrophil count less than 2000/mL, whereas 120 women (7%) had neutrophil counts less than 1000/mL at baseline. During 7.5 years of follow-up, and occurring on at least 1 occasion, a neutrophil count less than 2000/mL was documented in 79%, whereas neutrophil counts less than 1000/mL occurred in 31%.
FACTORS ASSOCIATED WITH DEVELOPMENT OF NEUTROPENIA
Among women who were free of neutropenia (neutrophil count >2000/mL) at their previous visit, transition models revealed that low CD4 cell count and high HIV-1 RNA viral load were significant independent risk factors for development of neutropenia during the 7.5-year follow-up period (Table 2). Compared with women with a CD4 cell count of 500/mL or higher, women with a CD4 cell count less than 200/mL were approximately 2 times more likely to develop neutropenia (odds ratio [OR], 1.82; 95% confidence interval [CI], 1.32-2.53). Relative to women with a viral load of less than 5000 copies/mL, women having 100 000 copies/mL or higher of HIV-1 RNA virus were 1.47 times more likely to develop neutropenia (OR, 1.47; 95% CI, 1.10-1.97). There was an increasing trend in the risk of development of neutropenia with decreasing CD4 cell count and increasing viral load (both P values for trend <.001).
Both anemia (OR, 1.45; 95% CI, 1.24-1.70) and thrombocytopenia (OR, 1.60; 95% CI, 1.27-2.02) were associated with a higher risk of developing neutropenia. The HIV-positive women using HAART without zidovudine were approximately 20% less likely to develop neutropenia (OR, 0.83; 95% CI, 0.67-1.01), whereas HAART regimens that contained zidovudine did not confer protection. In fact, the effect of HAART on protecting against development of neutropenia varied in a statistically significant manner, depending on whether zidovudine was included in the HAART regimen (P<.001) (Table 2). Use of combination ART without zidovudine was significantly protective against development of neutropenia (OR, 0.68; 95% CI, 0.51-0.92) (Table 2). The HIV-positive women who were pregnant at the study visit were also less likely to develop neutropenia (OR, 0.32; 95% CI, 0.14-0.72).
Women who were neutropenic at 2 visits before a given visit were approximately 3 times more likely (OR, 2.69; 95% CI, 2.26-3.21; P<.001) and at 3 visits before a given visit were 2.5 times more likely (OR, 2.55; 95% CI, 2.15-3.02; P<.001) to develop neutropenia again.
Using a conservative cut point for defining neutropenia (neutrophil count <1000/mL), low CD4 cell count, high HIV-1 RNA viral load, and the presence of anemia and/or thrombocytopenia maintained significance as a risk factor for the development of neutropenia. However, none of the ART-related variables were associated with the development of neutropenia (neutrophil count <1000/mL) (data not shown).
FACTORS ASSOCIATED WITH RESOLUTION OF NEUTROPENIA
Among women with a neutrophil count less than 2000/mL at their previous visit, current pregnancy, CD4 cell count, combination ART without zidovudine, and HAART with or without zidovudine were significantly associated with resolution of neutropenia (Table 2). Women who were currently pregnant at any given visit were 4.8 times more likely to correct neutropenia compared with those who were not pregnant (OR, 4.77; 95% CI, 2.18-10.40). Resolution of neutropenia was significantly less likely to occur if anemia or thrombocytopenia was also present.
Compared with women with a CD4 cell count of 500/mL or higher, women with a CD4 count less than 200/mL were 32% less likely to have resolution of neutropenia (P for trend <.001). There was no clear trend in the ORs for viral load categories of 5000 or more copies/mL. Compared with those without any ART, HIV-positive women receiving HAART with or without zidovudine and those receiving combination ART without zidovudine were significantly more likely to resolve neutropenia.
Women who were neutropenic 2 visits before a given visit were 49% less likely (OR, 0.51; 95% CI, 0.43-0.60; P<.001) and 3 visits before a given visit were 51% less likely (OR, 0.49; 95% CI, 0.41-0.58; P<.001) to experience resolution of neutropenia.
|
|
| |
| |
|
|
|