| |
Trizivir 96 weeks - impact on lipids: effect of sex & ethnicity
|
| |
| |
"A prospective, 96-week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters, lactate, and efficacy in antiretroviral-naive patients: effect of sex and ethnicity"
HIV Medicine
Volume 7 Page 85 - March 2006
PN Kumar1, A Rodriguez-French2, MA Thompson3, KT Tashima4, D Averitt5, PG Wannamaker6, VC Williams6, MS Shaefer6, GE Pakes6 and KA Pappa6 on behalf of the ESS40002 study team
1Georgetown University Medical Center, Washington, DC, USA, 2San Fernando Hospital, Panama City, Panama, 3ARCA, Atlanta, GA, USA, 4The Miriam Hospital, Providence, RI, USA, 5The Well Project, Charlottesville, VA, USA, and 6GlaxoSmithKline, Research Triangle Park, NC, USA
This study was funded by GlaxoSmithKline.
The results of this study were presented in part in Poster 27 at the 3rd International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, 23-26 October 2001, Athens, Greece; Abstract 33 at the 9th Conference on Retroviruses and Opportunistic Infections, 24-28 February 2002, Seattle, Washington; and Poster 709 and Oral Presentation 57 at the 2nd IAS Conference on HIV Pathogenesis and Treatment, 13-16 July 2003, Paris, France.
... In conclusion, at similarly effective doses, treatment of antiretroviral-naive patients with TZV twice daily over 96 weeks had no deleterious effect on LDL cholesterol, in contrast to COM/NFV or d4T/3TC/NFV twice daily. The lower likelihood of producing adverse LDL-cholesterol changes with TZV was particularly apparent in female and black patients....
"....In our study, in which the study population had a mean fasting LDL-cholesterol concentration within the normal range at baseline, no patient treated with TZV developed LDL-cholesterol levels >160 mg/dL at 96 weeks, whereas such LDL-cholesterol levels were observed in 13% of patients on d4T/3TC/NFV and 14% of those on COM/NFV....
.... This clinical trial is the first long-term study to test for statistical differences in lipid changes between sexes and among different ethnic groups following initiation of antiretroviral therapy. The study results showed that TZV twice daily may result in less pronounced increases in LDL cholesterol in women and black patients and in triglycerides in Hispanic patients than d4T/3TC/NFV, with the effects of COM/NFV being intermediate between the other two regimens....
At study week 96, LSM LDL cholesterol was very similar in women and men in the TZV group (94 versus 93 mg/dL), but higher in women treated with d4T/3TC/NFV (134 versus 126 mg/dL in men) and COM/NFV (121 versus 117 mg/dL in men).
... ESS40002 was conducted over 96 weeks to compare ABC/3TC/ZDV [as Trizivir (TZV); GlaxoSmithKline, Research Triangle Park, NC] with two double-NRTI/single-PI regimens with respect to the development of lipid and metabolic abnormalities and to monitor virological suppression and CD4 cell count changes in a population of antiretroviral-naive patients that included a substantial number of women and people from ethnic minorities...
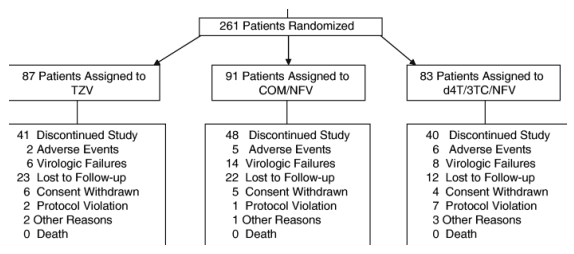
Of 254 patients enrolled (ITT population), 85 were randomized to TZV, 88 to COM/NFV, and 81 to d4T/3TC/NFV. The mean age of patients was 34 years in the TZV group, and 35 years in the other two groups (Table 1). The patient population was diverse with respect to sex (50% female) and ethnicity (40% black and 37% Hispanic American).
ABSTRACT
Objectives
To compare the lipid and metabolic effects, efficacy, and safety of twice-daily regimens of Trizivir (abacavir 300 mg/lamivudine 150 mg/zidovudine 300 mg triple nucleoside tablet; TZV), Combivir (lamivudine 150 mg/zidovudine 300 mg combination tablet; COM)+nelfinavir (NFV), and stavudine (d4 T)+lamivudine (3TC)+NFV.
Study design
An international, phase 4, open-label, parallel-group, 34-centre study was conducted in 254 non-diabetic, antiretroviral-naive, HIV-infected out-patients with an HIV-1 RNA level of >1000 HIV-1 RNA copies/mL and ≦200 000 copies/mL and a CD4 cell count of >50 cells/_L.
Methods
Patients were randomized 1 : 1 : 1 to TZV twice daily (n=85), COM/NFV 1250 mg twice daily (n=88), or d4T 40 mg+3TC 150 mg+NFV 1250 mg twice daily (n=81) for 96 weeks. Treatments were compared using analysis of covariance (ANCOVA) with regard to changes from baseline in fasting lipids in the total population and in sex and ethnic subgroups. The proportions of patients achieving HIV-1 RNA <50 and <400 copies/mL were compared using a 95% confidence interval (CI) on the difference between proportions.
Results
- The study population was diverse (50% female, 40% black and 37% Hispanic).
- Mean baseline low-density lipoprotein (LDL) cholesterol was 99 mg/dL, HIV-1 RNA was 4.43 log10 copies/mL and CD4 cell count was 355 cells/_L.
- At week 96, fasting LDL cholesterol changed minimally in the TZV group [least square mean (LSM) change from baseline, -8 mg/dL], but increased with d4T/3TC/NFV and COM/NFV (+29 and +19 mg/dL, respectively; P<0.001 versus TZV).
- Week 96 LDL-cholesterol levels were significantly lower in the TZV group than in the other two treatment groups in women and men and lower than in the d4T/3TC/NFV group in Hispanic and black patients.
- In black patients, the week-96 LSM change from baseline in LDL cholesterol was significantly less with TZV than with d4T/3TC/NFV (+1 vs+39 mg/dL; P=0.003).
- Total cholesterol>200 mg/dL occurred in a smaller proportion of patients receiving TZV (30%) compared with COM/NFV (50%) or d4T/3TC/NFV (60%; P=0.005 vs TZV).
- High-density lipoprotein (HDL) cholesterol did not change markedly with any treatment.
- Although triglycerides increased, they changed least in women and Hispanic patients receiving TZV.
- Virological and CD4 responses to the treatments were similar in the total population and in the subgroups.
- Diarrhoea was reported more often in the NFV arms and nausea in the ZDV arms.
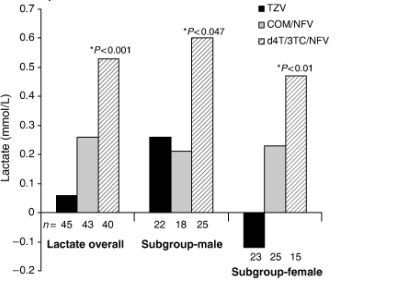
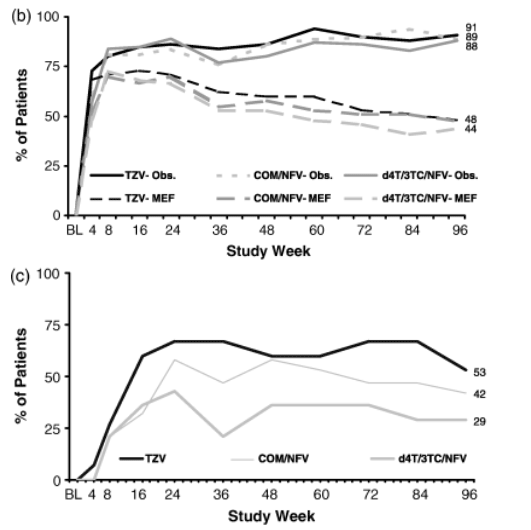
- At week 96, the ITT:observed analysis showed no significant differences amongst the TZV, COM/NFV and d4T/3TC/NFV groups in proportions of patients achieving <50 copies/mL [TZV, 78% (35 of 45); COM/NFV, 72% (34 of 47); d4T/3TC/NFV, 66% (27 of 41)] (Fig. 4a) or <400 copies/mL [TZV, 91% (41 of 45); COM/NFV, 89% (42 of 47); d4T/3TC/NFV, 88% (36 of 41)].
Conclusions
Over 96 weeks, TZV twice daily has significantly less effect on LDL cholesterol than COM/NFV or d4T/3TC/NFV twice daily, especially in women and black patients, and is associated with similar virological and CD4 responses.
RESULTS
Patient characteristics and disposition
More patients were recruited at study sites within the continental USA (186 randomized) than at other sites (Panama, 30; Dominican Republic, 21; Guatemala, 12; Puerto Rico, 12). Of 254 patients enrolled (ITT population), 85 were randomized to TZV, 88 to COM/NFV, and 81 to d4T/3TC/NFV. The mean age of patients was 34 years in the TZV group, and 35 years in the other two groups (Table 1). The patient population was diverse with respect to sex (50% female) and ethnicity (40% black and 37% Hispanic American). The ratios of women to men, and of each ethnic group to the others, were comparable among the treatment arms (differential statistical analysis not performed). At baseline, overall mean HIV-1 RNA was 4.43 log10 copies/mL and was significantly higher in men than in women (P=0.012) regardless of treatment arm; mean CD4 cell count was 355 cells/_L. The majority (81%) of the total study population had baseline plasma HIV-1 RNA levels >1000-100 000 copies/mL (viral load strata per treatment regimen shown in Table 1).
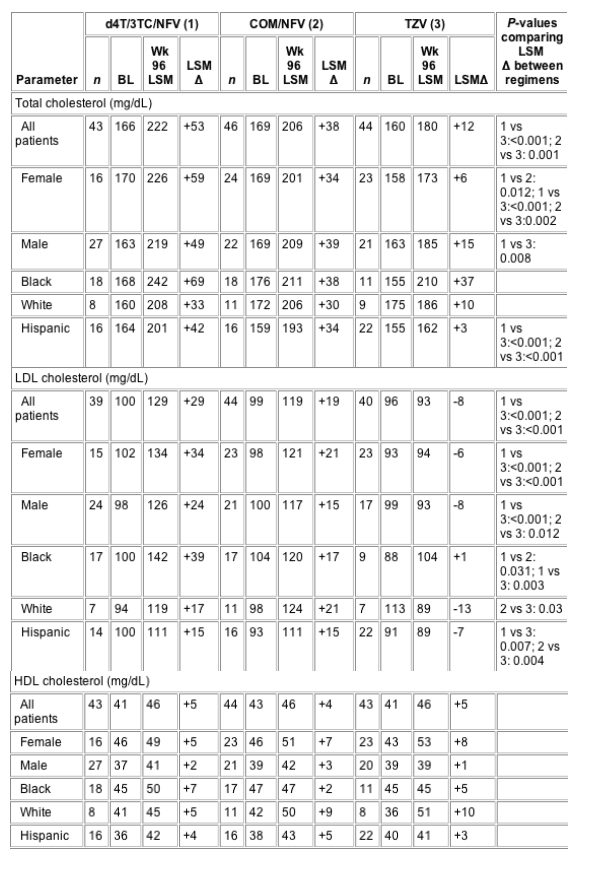
Baseline mean serum concentrations of total, LDL and HDL cholesterol, triglycerides, free fatty acids, lactate, glucose, insulin, and C-peptide were within normal limits. In the TZV, COM/NFV, and d4T/3TC/NFV groups, no significant differences were observed among treatments in baseline mean total cholesterol (overall P=0.158), LDL cholesterol (overall P=0.644), HDL cholesterol (overall P=0.533), or triglycerides (overall P=0.295) (Table 2). Regardless of treatment arm at baseline, women had significantly higher HDL-cholesterol levels (P<0.001) and lower triglycerides (P=0.001) than men and a significant difference among the ethnic groups in HDL cholesterol was observed (P=0.001), with black patients tending to have higher levels.
Approximately 50% of patients in each treatment group had discontinued the study prematurely by week 96 (Fig. 1). A greater proportion in the d4T/3TC/NFV and COM/NFV groups discontinued for treatment-related reasons (35% and 40%, respectively, versus 20% for TZV), most notably virological failure (20% and 29%, respectively, versus 15% for TZV). Of patients who discontinued prematurely for administrative reasons, a higher proportion in the TZV group dropped out because they were lost to follow up (56% versus 46% for COM/NFV and 30% for d4T/3TC/NFV), whereas a greater proportion in the d4T/3TC/NFV group did so because of a protocol violation (18% vs 5% for TZV and 2% for COM/NFV).
Changes in lipids
LDL cholesterol (see graphs immediately following text below)
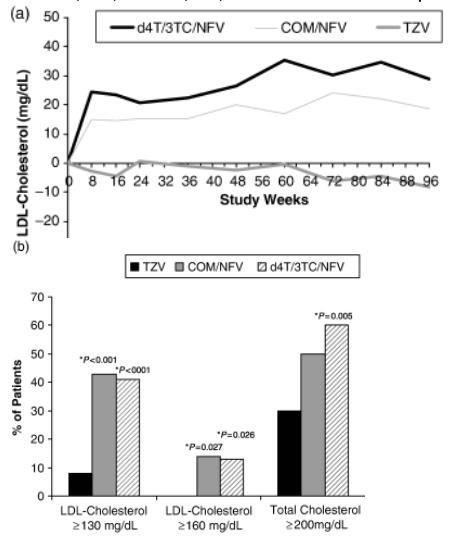
LDL cholesterol in the TZV group did not change markedly over the duration of the study. However, increases above baseline in LDL cholesterol in both the d4T/3TC/NFV and COM/NFV groups were greater than changes observed in the TZV group from the first postbaseline visit (week 8) onwards (Fig. 2a), with LSM change from baseline at week 96 being +29, +19 and -8 mg/dL, respectively (Table 2).
At week 96, only 8% (three of 40) of the TZV group had LDL-cholesterol levels ≥130 mg/dL compared with 41% (16 of 39) of the d4T/3TC/NFV group (P<0.001 versus TZV) and 43% of the COM/NFV group (P<0.001 versus TZV), and no patient in the TZV group had LDL cholesterol ≥160 mg/dL, compared with 13% (five of 39) of the d4T/3TC/NFV group (P=0.026 versus TZV) and 14% (six of 44) of the COM/NFV group (P=0.027 versus TZV) (Fig. 2b).
At study week 96, LSM LDL cholesterol was very similar in women and men in the TZV group (94 versus 93 mg/dL), but higher in women treated with d4T/3TC/NFV (134 versus 126 mg/dL in men) and COM/NFV (121 versus 117 mg/dL in men). LSM change from baseline in LDL-cholesterol levels was similar in women and men treated with TZV (-6 versus -8 mg/dL); the greatest LSM increase from baseline in LDL cholesterol was observed in women in the d4T/3TC/NFV group (+34 versus +24 mg/dL, P<0.001 versus TZV for both sexes), with smaller but still notable increases in the COM/NFV group [+21 versus +15 mg/dL, P<0.001 versus TZV (women) and P=0.012 versus TZV (men)].
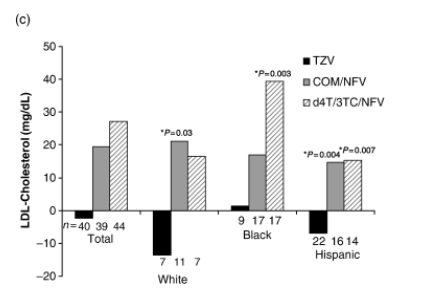
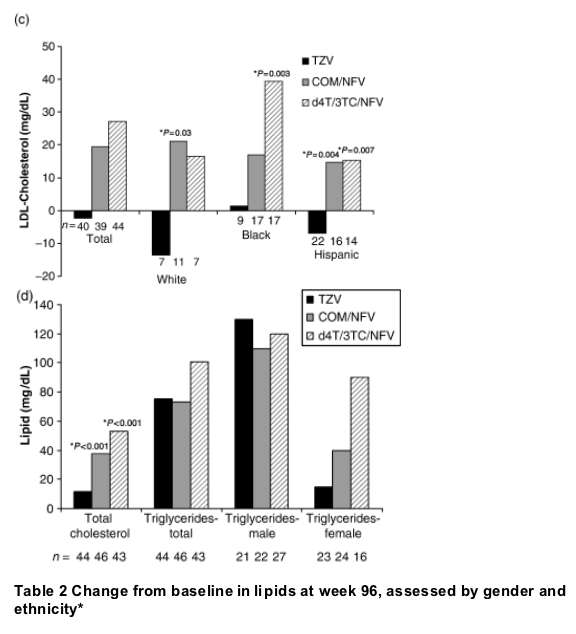
Black, Hispanic and white patients in both the d4T/3TC/NFV and COM/NFV groups experienced increases from baseline in LDL cholesterol over the course of the study, which generally plateaued after week 8. For every ethnic subgroup, these changes were significantly greater than those observed in the TZV group at all study visits between weeks 8 and 96. In black patients, the week-96 LSM elevation from baseline in LDL cholesterol was significantly lower with TZV than with d4T/3TC/NFV (+1 versus +39 mg/dL, P=0.003) (Fig. 2c).
Total cholesterol
At week 96, the LSM increase from baseline was significantly lower in the TZV group than in the d4T/3TC/NFV or COM/NFV group (+12 mg/dL versus +53 and +38 mg/dL, P<0.001) (Fig. 2d). Mean total cholesterol remained <200 mg/dL only in the TZV group (Table 2). Moreover, the proportion of patients with total cholesterol levels >200 mg/dL after 96 weeks of treatment was lower in the TZV group (30%) than in the COM/NFV group (50%) or the d4T/3TC/NFV group (60%; P=0.005 versus TZV) (Fig. 2b).
The analysis by sex showed that, at week 96, LSM increase from baseline in total cholesterol was lower in the TZV group than in the COM/NFV and d4T/3TC/NFV groups both in women (+6, +34 and +59 mg/dL, respectively) and in men (+15, +39 and +49 mg/dL, respectively). In women, but not in men, week-96 LSM change from baseline in total cholesterol differed significantly between the TZV and COM/NFV treatment groups (P=0.002) and between the COM/NFV and d4T/3TC/NFV groups (P=0.012). LSM total cholesterol remained <200 mg/dL in both women and men only in the TZV arm at weeks 48 and 96 (Table 2).
At week 96, LSM change from baseline did not differ between the TZV, COM/NFV and d4T/3TC/NFV groups in white or black patients, but did in Hispanic patients (+3, +34 and +42 mg/dL, respectively; P<0.001 for TZV versus d4T/3TC/NFV and for TZV versus COM/NFV). However, it is noteworthy that the sample sizes at week 96 were small (Table 2). In the TZV arm, the mean total cholesterol generally remained <200 mg/dL in all ethnic groups, whereas in the COM/NFV and d4T/3TC/NFV arms, this was observed only in Hispanic patients.
HDL cholesterol
HDL cholesterol did not change to a statistically significant degree with any treatment in either the total population or the sex and ethnic subgroups. The greatest LSM increases above baseline were observed in TZV-treated women (+8 mg/dL) and white patients (+10 mg/dL) (Table 2).
The mean total-cholesterol to HDL-cholesterol ratio, which was similar in the TZV, COM/NFV and d4T/3TC/NFV groups (4.23, 4.24 and 4.34, respectively) at baseline, remained below the baseline value in the TZV group at 96 weeks, at which time it had increased slightly in the COM/NFV group and risen to above 5 in the d4T/3TC/NFV group (mean 4.06, 4.65 and 5.16, respectively)
Triglycerides
Triglycerides increased in all treatment groups over the course of the study, including the period between weeks 48 and 96 (unlike LDL- and total-cholesterol levels). At week 96, LSM triglyceride levels were 211, 207 and 236 mg/dL in the TZV, COM/NFV and d4T/3TC/NFV groups, and the LSM change from baseline was +75, +72 and +101 mg/dL, respectively (no significant differences between treatments) (Fig. 2d). Women in the TZV group had the smallest increases in triglycerides during the study, with week-96 LSM change from baseline being +12 mg/dL compared with +39 mg/dL in the COM/NFV group and +89 mg/dL in the d4T/3TC/NFV group (Table 2). In contrast, men had larger increases from baseline in triglycerides; week-96 LSM change from baseline was +125, +108 and +113 mg/dL in the TZV, COM/NFV and d4T/3TC/NFV groups, respectively.
In the analysis by ethnicity, triglycerides increased in all treatment groups, with no significant between-treatment differences in black or white patients over 96 weeks. However, in Hispanic patients, the week-96 LSM increase from baseline in triglycerides was significantly lower in the TZV (P=0.002) and COM/NFV groups (P=0.021) than in the d4T/3TC/NFV group (+60, +83 and +167 mg/dL, respectively; Table 2).
Fig. 2 (a) Changes in fasting low-density lipoprotein (LDL) cholesterol in the three treatment groups over the whole study period. (b) Proportion of patients with LDL cholesterol ≥130 and ≥160 mg/dL at week 96. (c) Least square mean change from baseline in fasting LDL cholesterol according to ethnicity. (d) Change from baseline in total cholesterol and triglycerides. COM, Combivir (lamivudine 150 mg/zidovudine 300 mg combination tablet); TZV, Trizivir (abacavir 300 mg/lamivudine 150 mg/zidovudine 300 mg triple nucleoside tablet); d4T, stavudine; NFV, nelfinavir; 3TC, lamivudine. *P-value as compared with TZV.
*Baseline means are presented along with adjusted (least square) means at week 96.
Only P-values<0.05 are shown; all other P-values indicated no statistically significant differences.
All lipid values can be compared to the standards set forth in the 2001 NCEP Adult Lipid Guidelines: LDL cholesterol (mg/dL): <100 is optimal; 100-129 is near/above optimal; 130-159 is borderline high; 160-189 is high; ≥190 is very high; total cholesterol (mg/dL): <200 is desirable; 200-239 is borderline high; ≥240 is high; HDL cholesterol (mg/dL): <40 is low; ≥60 is high; triglycerides (mg/dL): <150 is normal; 150-199 is borderline high; 200-499 is high; ≥500 is very high. BL, baseline; COM/NFV, Combivir (lamivudine 150 mg/zidovudine 300 mg)+nelfinavir; d4T/3TC/NFV,
stavudine+lamivudine+nelfinavir; LSM, least squares mean; TZV, Trizivir (abacavir 300 mg/lamivudine 150 mg/zidovudine 300 mg); HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Changes in metabolic parameters
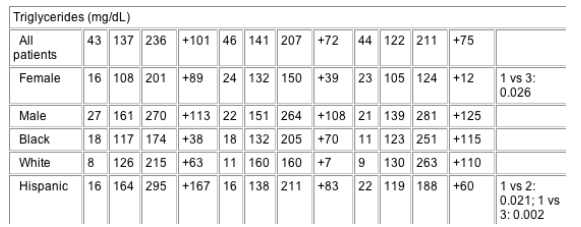
For serum fasting glucose, insulin and C-peptide, no differences amongst treatments were observed over 96 weeks in the total study population, between sexes, or between ethnic groups. Serum lactate did not differ between the TZV, COM/NFV, and d4T/3TC/NFV treatment groups at baseline (means, 1.13, 1.18 and 1.27 mmol/L, respectively), but it was significantly higher in the d4T/3TC/NFV group at week 48 (LSM, 2.02 mmol/L versus 1.44 mmol/L with COM/NFV and 1.30 mmol/L in TZV; P≦0.001) and week 96 (LSM, 1.63 mmol/L versus 1.38 mmol/L with COM/NFV and 1.23 mmol/L with TZV; P<0.02) (Fig. 3). The proportion of patients with lactate levels ≥2 mmol/L at two consecutive visits after baseline was lower in the TZV group (1%; one of 85) than in the COM/NFV (5%; four of 88) or d4T/3TC/NFV group (14%; 10 of 81). Similar elevations in lactate levels were seen across sex and ethnic subgroups.
Fig. 3 Least square mean change from baseline in serum lactate values observed at 96 weeks. COM, Combivir (lamivudine 150 mg/zidovudine 300 mg combination tablet); TZV, Trizivir (abacavir 300 mg/lamivudine 150 mg/zidovudine 300 mg triple nucleoside tablet); d4T, stavudine; NFV, nelfinavir; 3TC, lamivudine. *P-value as compared with TZV.
Efficacy
At week 96, the ITT:observed analysis showed no significant differences amongst the TZV, COM/NFV and d4T/3TC/NFV groups in proportions of patients achieving <50 copies/mL [TZV, 78% (35 of 45); COM/NFV, 72% (34 of 47); d4T/3TC/NFV, 66% (27 of 41)] (Fig. 4a) or <400 copies/mL [TZV, 91% (41 of 45); COM/NFV, 89% (42 of 47); d4T/3TC/NFV, 88% (36 of 41)].
The ITT:M=F analysis also showed no differences amongst treatments regarding proportions achieving HIV-1 RNA <50 copies/mL [TZV, 41% (35 of 85); COM/NFV, 39% (34 of 88); d4T/3TC/NFV, 33% (27 of 81)] (Fig. 4a) or <400 copies/mL [TZV, 48% (41 of 85); COM/NFV, 48% (42 of 88); d4T/3TC/NFV, 44% (36 of 81)] (Fig. 4b). In patients whose baseline viral load was <100 000 copies/mL, the proportion with HIV-1 RNA <50 copies/mL at week 96 was 39% (27 of 70) with TZV, 38% (26 of 69) with COM/NFV, and 34% (23 of 67) with d4T/3TC/NFV. The proportion of patients with baseline HIV-1 RNA levels>100 000 and <200 000 copies/mL who achieved HIV-1 RNA <50 copies/mL at week 96 was greater in the TZV group than in the d4T/3TC/NFV or COM/NFV group [53% (eight of 15) versus 29% (four of 14) and 42% (eight of 19), respectively; Fig. 4c].
Sex and ethnicity did not affect the proportions of patients achieving HIV-1 RNA <50 or <400 copies/mL or LSM reduction in HIV-1 RNA from baseline, although 10-20% more Hispanic than white or black patients treated with TZV had undetectable viral loads at week 96. Virological failure (confirmed HIV-1 RNA >2000 copies/mL) occurred in 6% of patients (five of 85) on TZV, 11% of patients (10 of 88) on COM/NFV, and 7% of patients (six of 81) on d4T/3TC/NFV. In patients with virological failure, median times to confirmed failure (and, thus, treatment discontinuation) were similar in the three treatment arms (256, 272 and 266 days, respectively).
CD4 cell counts increased steadily in all treatment groups over the duration of the study. At week 96, the LSM CD4 cell counts were 619, 625 and 643 cells/_L, respectively, with the LSM change from baseline being +265, +270 and +289 cells/_L, respectively (no significant differences). Sex and ethnicity did not affect the magnitude of CD4 increases from baseline over the course of the study.
Fig. 4 Virological response in terms of proportion of patients achieving HIV-1 RNA <50 copies/mL (a) and <400 copies/mL (b) and proportion of patients with baseline HIV-1 RNA >100 000 copies/mL but <200 000 copies/mL who achieved <50 copies/mL over the study period (c) COM, Combivir (lamivudine 150 mg/zidovudine 300 mg combination tablet); TZV, Trizivir (abacavir 300 mg/lamivudine 150 mg/zidovudine 300 mg triple nucleoside tablet); d4T, stavudine; NFV, nelfinavir; 3TC, lamivudine; Obs, observed analysis; MEF, missing equals failure analysis.
Treatment-emergent viral resistance
The criteria for virological failure were met by four patients on TZV, 10 on COM/NFV, and six on d4T/3TC/NFV. Mutations that were detected by genotyping were typical of those previously reported with the components of the regimens. In the TZV group, reverse transcriptase (RT) mutations observed were D67N (one patient) and M184 V (one patient), and protease (PRO) mutations were D30N (one patient who was switched to the COM/NFV regimen) and M36I (one patient). In the COM/NFV group, RT mutations observed were K103N (one patient who had efavirenz added to the regimen), V118I (one patient), M184V (six patients), and R211K (one patient), and PRO mutations were K20I (one patient), D30N (one patient), M46L (one patient), A71V (one patient), and L90 M (two patients). In the d4T/3TC/NFV group, RT mutations seen were M184V (five patients) and L189I (one patient), and PRO mutations were D30N (two patients), M36I (one patient), A71T (one patient), N88D (one patient), N88S (one patient), and L90 M (two patients).
Body fat redistribution and bone mineral density changes
DEXA findings at week 96 showed no significant differences in changes from baseline in total fat, left and right arm fat, left and right leg fat, trunk fat, trunk to extremity fat ratio, trunk to total fat ratio, or leg to total fat ratio among the treatment groups. This result may have been a consequence of the small number of patients with complete data available (n=10 in the TZV group, 12 in the COM/NFV group, and 13 in the d4T/3TC/NFV group). Mean per cent changes from baseline in waist-to-hip ratio were similar in the TZV, COM/NFV and d4T/3TC/NFV groups, varying from 5 to 9% in women and from 0 to 7% in men. Body image questionnaire results, which were available at week 96 from 47 patients on TZV, 46 on COM/NFV, and 41 on d4T/3TC/NFV, showed no consistent trends to differentiate the three treatment groups.
A total of 62 patients had bone mineral density data available at baseline, and 15 (five in each treatment arm) at week 96. Bone mineral density changed minimally over the course of the study. No consistent trends were observed in the TZV, COM/NFV and d4T/3TC/NFV groups regarding t-scores (mean at week 96 versus baseline: -0.19 versus -0.03; -0.75 versus -1.27; and -0.51 versus -0.32, respectively) or z-scores (mean at week 96 versus baseline: -0.03 versus +0.72; -0.49 versus -1.22; and -0.45 versus -0.22, respectively) to differentiate the effects of the three treatments in the total study population.
Safety
The most frequently reported treatment-related adverse events (≥5% of patients) in all three groups were gastrointestinal in nature (Table 3). Diarrhoea was reported more frequently in patients treated with COM/NFV or d4T/3TC/NFV than in patients treated with TZV (51% and 48%, respectively, versus 6% with TZV), and nausea more frequently with TZV and COM/NFV (30% and 32%, respectively, versus 17% with d4T/3TC/NFV). Frequently reported non-gastrointestinal-related adverse events were fatigue and headache. Serious adverse events, which included three cases of suspected ABC-related hypersensitivity reaction, are described in Table 3.
Table 3 Drug-related* adverse events that occurred in ≥5% of patients*
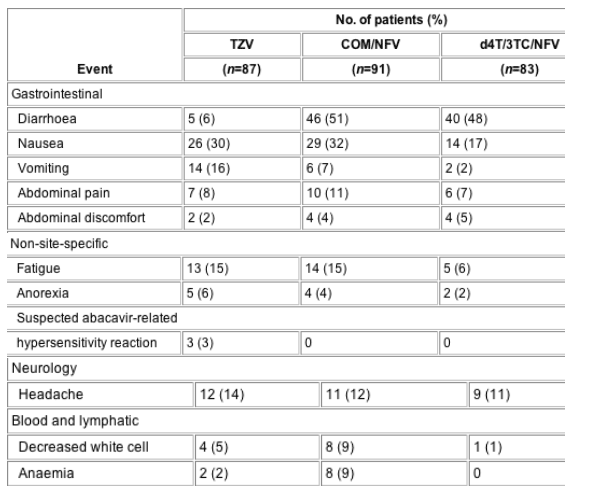
Drug-related adverse events leading to premature discontinuation from the study were experienced by the following patients in the treatment groups (individual patients could experience more than one of these adverse events): two patients on TZV [abdominal pain (one), nausea (one), vomiting (one), headaches (one), allergic reaction to medicinal substance (one), pain (one), throat signs and symptoms (one), and chest symptoms (one)]; five on COM/NFV [diarrhoea (two), nausea (one), abdominal pain (one), protozoal gastrointestinal infections (one), anaemia (one), primary malignant blood and lymphatic neoplasia (one), palpitations (one), anxiety (one), and depressive disorders (one)]; and six on d4T/3TC/NFV (diarrhoea (one), nausea (one), vomiting (one), peripheral neuropathy (one), hepatitis (one), pancreatitis (one), rashes (two), and acidosis (one)].
Serious adverse events, which may not have been attributable to drug treatment, were reported by 12 patients receiving TZV [suspected abacavir-related hypersensitivity reaction (three), appendicitis (one), gastritis (one), protozoal gastrointestinal infections (one), drug abuse and withdrawal (one), depressive disorders (one), suicide/attempted suicide (two), bacterial respiratory infections (one), ear, nose and threat infections (one), and abortion and stillbirth (one)]; four receiving COM/NFV [abdominal pain (one), depressive disorders (one), bronchitis (one) and chest symptoms (one)]; and seven receiving d4T/3TC/NFV [duodenal ulcers (one), nausea (one), vomiting (one), drug abuse and withdrawal (one), depressive disorders (one), lower respiratory failure (two), and lower respiratory infections (two)].
DISCUSSION
The results of this clinical trial indicate that over a 96-week period triple NRTI treatment with TZV twice daily has a significantly smaller effect on LDL cholesterol than COM/NFV or d4T/3TC/NFV twice daily, and is less likely to result in clinically significant LDL-cholesterol elevation. Current National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III guidelines emphasize the importance of maintaining patients with elevated LDL-cholesterol levels plus 0-1 CHD risk factors at an LDL cholesterol <160 mg/dL [7,12]. In our study, in which the study population had a mean fasting LDL-cholesterol concentration within the normal range at baseline, no patient treated with TZV developed LDL-cholesterol levels >160 mg/dL at 96 weeks, whereas such LDL-cholesterol levels were observed in 13% of patients on d4T/3TC/NFV and 14% of those on COM/NFV. Clinically important effects of TZV on LDL cholesterol and other lipids were not expected because previous direct comparative clinical trials in antiretroviral-naive patients with baseline viral loads <100 000 copies/mL demonstrated that ABC/3TC/ZDV does not produce the marked lipid-increasing effects observed with NFV- or indinavir-containing HAART [9-11]. Moreover, studies evaluating metabolic effects following replacement of the PI in regimens with ABC have actually shown resolution of PI-induced lipid elevations [13,14]. The individual components of the TZV regimen-3TC, ZDV and ABC-have few known lipogenic properties [15-17].
This clinical trial is the first long-term study to test for statistical differences in lipid changes between sexes and among different ethnic groups following initiation of antiretroviral therapy. The study results showed that TZV twice daily may result in less pronounced increases in LDL cholesterol in women and black patients and in triglycerides in Hispanic patients than d4T/3TC/NFV, with the effects of COM/NFV being intermediate between the other two regimens. This study confirmed previous lipid research in non-HIV-infected subjects showing generally higher HDL-cholesterol levels, but similar LDL-cholesterol levels, in women compared with men and in black adults compared with white adults [7]. It also confirmed that initiating treatment-naive patients on regimens containing the PI NFV prompts long-term increases in the total/HDL cholesterol ratio; similar elevations in this ratio also have been observed with indinavir-containing regimens over 48 weeks [18].
The finding that TZV twice daily did not have a significant effect on LDL cholesterol and reduced the total/HDL cholesterol ratio below baseline has important implications for treatment. The TZV regimen would be appropriate as a treatment option for patients diagnosed with HIV infection who have pre-existing hyperlipidaemia and CHD risk factors. Women with hyperlipidaemia have been shown to be prescribed antihyperlipidaemic drugs less often in clinical practice than men; thus, any treatment option that avoids exacerbating lipid abnormalities in women would be desirable. The HDL-cholesterol results of this study are generally consistent with previous research with the study drugs [15-17]. None of the regimens resulted in a significant change in HDL cholesterol, although week-96 results showed that the largest LSM increase from baseline was observed in women treated with TZV (+8 mg/dL). The HDL-cholesterol findings with the NFV-containing regimens in this study differed from those of APV30002 (SOLO). In the latter clinical trial, 327 antiretroviral-naive patients were treated with NFV 1250 mg twice daily in combination with ABC 300 mg twice daily and lamivudine 150 mg twice daily, and a 7.7 mg/dL increase in HDL cholesterol was observed at week 48 [19,20]. However, this occurred without improvement in the total/HDL cholesterol ratio.
Increases in lipid levels following initiation of the NFV-containing regimens were expected because LDL cholesterol, total cholesterol, and triglycerides have all been shown to increase within a few weeks of adding NFV to treatment regimens [21-23]. The Swiss HIV Cohort Study showed that, over 470 days, initially normolipidaemic HIV-infected patients who were treated with NFV experienced a mean increase in total cholesterol of 46 mg/dL, and that 33% developed total cholesterol levels exceeding 240 mg/dL [23]. Patients taking NFV also have been shown to have a higher coronary artery calcium score compared with patients on non-PI-containing regimens, suggesting that NFV use may be more likely to be associated with accelerated atherosclerosis, and hence greater risk of coronary artery disease (CAD) [24]. The increase in lipids observed in the d4T/3TC/NFV group was greater than that observed in the COM/NFV group, confirming the results of earlier clinical trials that showed that d4T induces increases in cholesterol and triglycerides that are additional to those caused by concurrently administered PIs [25,26]. The value of TZV with respect to facilitating adherence and enhancing patient satisfaction over more complex HAART regimens has been reported elsewhere [27,28].
This study demonstrated no significant sex or ethnic group effects with respect to clinical response to the treatment regimens that were evaluated. Week-96 data confirmed that virological suppression and increases in CD4 cell counts observed at week 48 were maintained through week 96. The proportion of patients achieving HIV-1 RNA <400 copies/mL and <50 copies/mL with TZV in the ITT:observed analysis at week 48 was comparable to that reported previously with the ABC/3TC/ZDV triple nucleoside combination in other clinical trials that have been conducted over 48 weeks [9,10].
The efficacy results of TZV in ESS40002 are consistent with the interim efficacy findings with TZV in ACTG5095, a currently ongoing double-blind, placebo-matched clinical trial that is similarly evaluating an ethnically diverse population of antiretroviral-naive patients (36% black and 21% Hispanic) [8]. The ITT:switch included analysis in the latter study showed that at 48 weeks 74% of patients treated with TZV achieved HIV-1 RNA <200 copies/mL and mean CD4 cell counts increased above baseline by 174 cells/_L. However, cross-comparisons of the results of ESS40002 with those of ACTG5095 must be tempered by the fact that ACTG5095 evaluated a population with higher mean baseline HIV-1 RNA (4.85 log10 copies/mL) and lower mean CD4 cell count (238 cells/_L), and utilized a double-blind design requiring patients to take 7 pills/day of the TZV (including five placebos) instead of the 2 pills/day prescribed in clinical practice. It is to be noted that the National Institute of Allergy and Infectious Diseases Data and Safety Monitoring Board (DSMB) overseeing ACTG5095 discontinued the TZV arm after an average of 32 weeks because the proportion of the 167 antiretroviral-naive patients who had experienced virological failure (two successive HIV-1 RNA levels ≥200 copies/mL at week 16 or later) up to that time-point was larger in the TZV arm than in the two comparator arms (Combivir +efavirenz or TZV+efavirenz) combined (21% versus 10%) [29].
In this study, the TZV regimen was well tolerated over 96 weeks and the safety profile did not change over time. Thus, the incidence of adverse events in the TZV group at 96 weeks was comparable to that reported in other studies that have evaluated ABC/3TC/ZDV over 48 weeks [13,14]. The greater elevation of serum lactate in the d4T/3TC/NFV group is consistent with earlier studies showing d4T to be associated with most NRTI-related cases of hyperlactataemia, which may be related to the greater mitochondrial toxicity profile of this thymidine compared to other NRTIs [30].
This study was well designed to evaluate how lipid and metabolic parameters, clinical response and the safety profile could change over 1 year past the typical 48-week clinical trial period. The use of a large population including female, black and Hispanic patients was crucial to ensure that study participants reflected the population in whom HIV infection cases are currently increasing most rapidly. Although we did not assess patient overall CHD risk factors, such an assessment might have been valuable. We attempted to collect data on lipodystrophy in this study, but it was logistically difficult to comply with the procedure. The stringent requirements of DEXA scanning, especially those pertaining to timing and scheduling of DEXA, may have contributed to the higher than normal dropout rate of patients in this study. Two other limitations of the study were that adherence was not evaluated or compared between the treatment regimens; and conclusions about combined race and sex effects on lipids could not be drawn because of sample size issues.
In conclusion, at similarly effective doses, treatment of antiretroviral-naive patients with TZV twice daily over 96 weeks had no deleterious effect on LDL cholesterol, in contrast to COM/NFV or d4T/3TC/NFV twice daily. The lower likelihood of producing adverse LDL-cholesterol changes with TZV was particularly apparent in female and black patients. This low pill burden regimen maintained virological suppression over 96 weeks and was well tolerated. Overall, the use of TZV would be optimal when considering treating HIV-infected patients with pre-existing hyperlipidaemia, possibly preventing exacerbation of the condition and obviating the need for antihyperlipidaemic agents and their incumbent drug interactions.
INTRODUCTION
Women and ethnic minorities are often underrepresented in clinical trials of antiretroviral drugs despite the fact that the number of new cases of HIV infection and AIDS has increased disproportionately in these groups compared with the total HIV-infected population [1-4]. The Centers for Disease Control and Prevention (CDC) have documented that the proportion of AIDS cases among women and adolescent girls (aged>13 years) has more than tripled from 8% in 1986 to 27% in 2003 [3]. Black people currently constitute only 12% of the US population, but they account for approximately half (50.5%) of all HIV/AIDS cases in the USA [3]. In 2003, the incidence of AIDS per 100 000 population in the USA was nearly 10-fold higher in black people than in white people (58.2 versus 6.1) and over 3-fold higher in Hispanic people (20.0) [3]. The clinical efficacy and safety findings of clinical trials that have evaluated predominantly white males may not be applicable to HIV-infected women and non-white ethnic groups. Women tend to have lower HIV-1 RNA levels and higher CD4 cell counts than men at the time of diagnosis [4].
When administered on a long-term basis, highly active antiretroviral therapy (HAART) regimens, especially those containing protease inhibitors (PIs), can result in hyperlipidaemia, insulin resistance and fat maldistribution characterized by abdominal obesity [5]. Further, HAART-associated hyperlipidaemia may increase the risk of coronary heart disease (CHD) in HIV-infected patients [6]. Risk factors for CHD appear to vary with sex and ethnicity. Cardioprotective high-density lipoprotein (HDL)-cholesterol concentrations are generally higher in premenopausal women and oestrogen-receiving postmenopausal women than in men, possibly accounting for the 10- to 15-year later onset of CHD in women [7]. Black and Hispanic people also tend to have higher HDL-cholesterol levels than white people, but generally have a higher prevalence of multiple CHD risk factors (hypertension, left ventricular hypertrophy, diabetes mellitus, cigarette smoking, obesity, and physical inactivity) [7]. The latter are believed to account for black people having the highest overall CHD mortality rate and the highest out-of-hospital coronary death rates of any ethnic group in the US [7].
In an effort to prevent long-term hyperlipidaemic and metabolic sequelae of antiretroviral treatment, clinicians have investigated combination regimens that have the least effect on lipids and other metabolic parameters with the greatest antiviral efficacy. In antiretroviral-naive patients with baseline viral loads <100 000 HIV-1 RNA copies/mL, a triple combination of the nucleoside reverse transcriptase inhibitors (NRTIs) abacavir (ABC) 300 mg, lamivudine (3TC) 150 mg, and zidovudine (ZDV) 300 mg, administered twice daily, was shown in one clinical trial to reduce HIV-1 RNA and increase CD4 cell counts over 48 weeks at least as well as nelfinavir (NFV)/3TC/ZDV, without prompting the significant lipid increases seen with the latter regimen [8]. In two other comparative trials, ABC/3TC/ZDV was as effective as indinavir (IDV)/3TC/ZDV over 48 weeks (intent-to-treat: switch or missing data=failure analyses), and did not significantly affect serum cholesterol or triglycerides [9,10]. Although ABC/3TC/ZDV was found to be less potent than two efavirenz (EFV)-containing regimens in one large-scale controlled study [8], there remains interest in the possibility that use of this particular triple nucleoside combination may be beneficial in certain subpopulations because of its lower likelihood of producing adverse metabolic effects and its ease of administration. To date, clinical studies evaluating the lipid and metabolic effects of HAART generally have not included study populations that have been diverse with respect to sex and ethnicity, and few have monitored patients for more than 1 year. In view of this, protocol ESS40002 was conducted over 96 weeks to compare ABC/3TC/ZDV [as Trizivir (TZV); GlaxoSmithKline, Research Triangle Park, NC] with two double-NRTI/single-PI regimens with respect to the development of lipid and metabolic abnormalities and to monitor virological suppression and CD4 cell count changes in a population of antiretroviral-naive patients that included a substantial number of women and people from ethnic minorities.
Methods
Patient selection
Female out-patients who were non-pregnant and non-lactating and male out-patients were eligible for study enrolment if they were at least 18 years of age; had HIV-1 infection (documented by HIV-1 antibody enzyme-linked immunosorbent assay and confirmed by western blot test of HIV-1 antibody, or positive HIV-1 blood culture, positive HIV serum antigen, or plasma viraemia); had a CD4 cell count >50 cells/_L; had a plasma HIV-1 RNA >1000 copies/mL but ≦200 000 copies/mL; were naive to antiretroviral therapy or of limited experience [≦1 week of treatment with 3TC or a PI, ≦4 weeks of treatment with other NRTIs, and no history of treatment with a nonnucleoside reverse transcriptase inhibitor (NNRTI)], and were not receiving antihyperlipidaemic or antidiabetic medications.
Study design and treatment
This phase 4, parallel-group, active control, randomized, open-label, multicentre trial was conducted between 25 October, 1999 and 3 September, 2002 at 34 out-patient sites in the USA, Puerto Rico, Guatemala, the Dominican Republic and Panama. A national, regional, or investigational centre Ethics Committee or Institutional Review Board approved the study protocol at each site. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki (October 1996). To participate in the study, all patients had to provide written informed consent.
Screening visit
At the screening visit, which occurred within 14 days pre-study, eligibility for the study was determined by having study candidates undergo a medical history, physical examination, CDC classification, and assessments of clinical chemistry, haematology, _-human chorionic gonadotropin (women of childbearing age only), viral load, and CD4 cell count. Plasma HIV-1 RNA levels were measured in blood samples using both the Roche Amplicor PCR Standard 1.0 assay [lower limit of quantification (LLOQ) 400 copies/mL] and the Roche PCR assay Amplicor HIV-1 Monitor UltraSensitive, Version 1.0 (LLOQ 50 copies/mL) (both assays from Roche Diagnostics, Branchburg, NJ). CD4 cell counts were determined by flow cytometry.
On-study visits
Patients meeting entry criteria were randomized 1:1:1 to:
- ABC/3TC/ZDV [given initially as one tablet of Combivir (COM; 3TC 150 mg/ZDV 300 mg combination tablet; GlaxoSmithKline) plus one tablet of ABC (Ziagen ; GlaxoSmithKline), and later as Trizivir (TZV; ABC 300 mg/3TC 150 mg/ZDV 300 mg combination tablet; GlaxoSmithKline)] twice daily;
- COM plus NFV 1250 mg twice daily (COM/NFV); or
- stavudine (d4T) 40 mg plus 3TC 150 mg plus NFV 1250 mg twice daily (d4T/3TC/NFV).
Patients in the COM/NFV group received each dose of NFV as five 250 mg tablets of Viracept (Agouron, La Jolla, CA), and patients in the d4T/3TC/NFV group weighing ≥60 kg received each dose of d4T as one 40 mg capsule of Zerit (patients weighing <60 kg received one 30 mg capsule twice daily) (Bristol-Myers Squibb, Princeton, NJ) and each dose of 3TC as 150 mg of Epivir (GlaxoSmithKline). Patients satisfying all enrolment criteria were stratified at randomization into two groups based on their screening plasma HIV-1 RNA level: <1000-100 000 copies/mL or >100 000-200 000 copies/mL.
Patients in the COM/NFV group received each dose of NFV as five 250 mg tablets of Viracept (Agouron), and patients in the d4T/3TC/NFV group weighing ≥60 kg received each dose of d4T as one 40 mg capsule of Zerit (patients weighing <60 kg received one 30 mg capsule twice daily) (Bristol-Myers Squibb, Princeton, NJ) and each dose of 3TC as 150 mg of Epivir (GlaxoSmithKline). Patients satisfying all enrolment criteria were stratified at randomization into two groups based on their screening plasma HIV-1 RNA level: <1000-100 000 copies/mL or >100 000-200 000 copies/mL.
During the study, patients visited their study site clinic at baseline (day 1), at weeks 4, 8, 16 and 24, and every 12 weeks thereafter until week 96 or withdrawal.
Assessment of lipids and metabolic parameters
The primary endpoint of the study was change from baseline in LDL cholesterol. Complete fasting lipid panels and plasma glucose concentrations were obtained from blood samples at baseline and at study weeks 4, 8, 16 and 24, and every 12 weeks thereafter until week 96 or withdrawal. Secondary lipid-related and metabolic endpoints included HDL cholesterol, total cholesterol and triglycerides, as determined at the above time-points, and fasting serum insulin, C-peptide, free fatty acids, and lactate concentrations, as determined at baseline and at study weeks 24, 48, 72 and 96 (or at the time of withdrawal). The study protocol did not stipulate any change in diet in patients whose lipids increased during the study.
Efficacy assessment
Changes in HIV-1 RNA levels and CD4 cell counts were secondary endpoints in this study. Plasma HIV-1 RNA levels and CD4 counts were measured in blood samples at all study visits. All HIV-1 RNA measurements were performed centrally by batch by Covance Inc. (Princeton, NJ). Virological failure was defined as HIV-1 RNA >2000 copies/mL on two occasions at least 2-4 weeks apart (standard at the time this study was designed). Plasma samples for resistance testing were collected at the baseline visit and, in patients who experienced virological failure, at the time of failing. Genotypic and phenotypic analyses of the HIV isolates were performed using the Virco Antivirogram assay and Virtual Phenotype system (Virco, Durham, NC), respectively.
Assessment of fat redistribution and bone mineral density
Fat redistribution and bone mineral density changes were secondary study endpoints recorded in a subset of patients (those enrolled at study sites equipped to assess these parameters). Fat redistribution was determined by measurement of per cent change in body fat levels using dual-energy X-ray absorptiometry (DEXA), waist-to-hip ratio measurements, and a body image questionnaire at baseline, at weeks 24, 48, 72, and 96, or at withdrawal. The body image questionnaire was developed by Glaxo Wellcome (Research Trangle Park, NC) and was a nonvalidated instrument. Clinical evidence of fat redistribution was defined as a 5% or greater change from baseline in per cent body fat in at least one of the following areas: trunk (5% increase), leg or arm (5% decrease); or a 25% or greater decrease in total body fat.
Bone mineral density changes were assessed by t-scores and z-scores at baseline and at study weeks 24, 48, 72, and 96.
Safety
The frequency and severity of all clinical and laboratory adverse experiences and the possibility of these being drug-related were assessed by investigators at each study visit.
Statistical analysis
A sample size of 45 patients per treatment arm was estimated to have 80% power to detect a difference of 25 mg/dL in LDL cholesterol between treatment groups using a two-group t-test with a two-sided significance level of 0.049. However, enrolment of a larger population was deemed necessary to accommodate the loss of patients as a result of dropouts expected over the long (96-week) study duration. The primary efficacy population was the intent-to-treat (ITT) population, which consisted of all patients who were randomized into the study and had postbaseline data. Analysis of covariance (ANCOVA) was used to compare least squares means (LSM) between treatment groups. The ANCOVA model included factors for treatment group, HIV-1 RNA strata and sex, and a covariate for the baseline value of the outcome variable of interest. The LSM represents the mean value adjusted for the average value of the covariate among the treatment groups. LSMs of change from baseline in serum lipids [total, LDL, very low-density lipoprotein (VLDL) and HDL cholesterol, and triglycerides], free fatty acids, C-peptide, glucose, insulin, and lactate were reported. Subgroup analyses of the primary endpoint (LDL cholesterol) and secondary endpoints were conducted by sex and race. No statistical analysis was performed to assess differences in sex ratio between treatment groups.
HIV-1 RNA data were evaluated by an ITT:observed analysis, in which only available assessments were used (no imputation for missing values), regardless of whether the patient was still receiving their original therapy, and an ITT:M=F analysis, in which all missing values were considered as failure. Proportions of patients with HIV-1 RNA <400 copies/mL and <50 copies/mL (ITT:observed and ITT:M=F) were compared between treatment groups with a 95% confidence interval (CI) on the difference between proportions.
Differences between treatment arms in the incidence of treatment-related adverse events by body system were evaluated by Fisher's exact test. A P-value of ≦0.05 was considered statistically significant.
|
|
| |
| |
|
|
|