| |
Poly-L-Lactic Acid for Facial Lipoatrophy - 2 years Followup
|
| |
| |
"Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy, 2 Years Follow-up"
HIV Medicine
Volume 7 Page 181 - April 2006
GJ Moyle1, S Brown1, L Lysakova2 and SE Barton1
ABSTRACT
Objective
We evaluated the long-term safety and efficacy of injectable poly-L-lactic acid (PLLA) for the correction of facial lipoatrophy.
Methods
This was a randomized, open-label, comparative, single-centre study of injected PLLA in patients with HIV-related facial lipoatrophy. Thirty subjects were randomized to immediate or delayed PLLA treatments, administered as three sets of bilateral injections, 2 weeks apart, into the deep dermis above the buccal fat pad. Week 24 results have been published previously (Moyle et al, HIV Medicine 2004, Vol. 5, pp. 82-87). Long-term efficacy was assessed at a recall visit using visual analogue scales (VASs) to record patient satisfaction, and by the Hospital Anxiety and Depression Scale (HADS). Patients also reported any adverse events (AEs) during the treatment period and at the recall visit.
Results
Twenty-seven patients returned for the recall visit, a minimum of 18 months post final study treatment. Fourteen of these patients were excluded from the recall visit because of additional treatment with PLLA. Improvements in VAS scores for facial appearance were sustained from baseline to the recall visit in both randomization groups (P<0.05 and P<0.001). Trends in improvement in HADS scores were also noted, with patients in the delayed group experiencing significant improvements in depressive symptoms (P<0.05). One case of injection-site induration and nine cases of injection-site nodules were noted at the recall visit, none of which was described as serious or severe.
Conclusions
Physical and psychological benefits of PLLA are sustained over at least 18 months. Delayed AEs include mild nodularity at the treatment site.
Introduction
Facial lipoatrophy can have a profound effect on patients with HIV infection. Problems include erosion of self-image and self-esteem, which can translate into personal and social relationship difficulties. Unlike many other symptoms of HIV infection or HIV-associated lipodystrophy syndrome, facial lipoatrophy may force patients to unmask their HIV status; more generally it suggests that an individual, who is otherwise healthy, is unwell [1]. With predictable consequences, these quality-of-life issues have been shown to reduce adherence to antiretroviral medication [2,3].
The only effective approach to management of lipoatrophy that has demonstrated benefit in randomized controlled clinical trials is switching therapy away from a thymidine analogue [4-6]. Not only is this option not available to all patients, but the statistically significant improvement in peripheral fat mass detected by dual-energy X-ray absorptiometry do not result in fat gains that were evident to patients and physicians, even 2 years after switching.
Two substantial clinical studies have demonstrated that injectable poly-L-lactic acid (PLLA; Sculptra/New-Fill; Dermik Laboratories, Berwyn, PA, USA) is well tolerated and effective at restoring facial volume in patients with HIV-related facial lipoatrophy [7,8]. The study by Moyle et al (2004) investigated the short-term (up to 24 weeks) safety and efficacy of PLLA injections for the cosmetic management of facial lipoatrophy [8].
In the initial part of that study, HIV-positive subjects (n=30) with facial lipoatrophy were randomized to an immediate group and a delayed group to receive bilateral PLLA injections at weeks 0, 2 and 4, and weeks 12, 14 and 16, respectively. PLLA was administered as three sets of injections, 2 weeks apart, into the deep dermis above the buccal fat pad. The study permitted all patients to be treated, but the delayed group acted as a control for the immediate group. Adverse events (AEs) were reported at each visit and at weeks 12 and 24, and at a recall visit 18 months post-treatment. Changes in laboratory values (CD4 count, viral load and lactate level) were also noted during the 24-week study period. Treatment efficacy was assessed at the recall visit with a visual analogue scale (VAS) recording patient satisfaction, and by administration of the Hospital Anxiety and Depression Scale (HADS) [8]. Here we report the long-term outcomes for patients from this study who consented to return for a recall visit, approximately 2 years after randomization.
Discussion
The safety data presented here suggest that PLLA has a favourable long-term safety and efficacy profile. Over 2 years, no serious or severe side effects were reported. As indicated by the significant improvement in VAS scores from baseline, and the trend for improvements in HADS scores, the positive results achieved with PLLA noted at 24 weeks [8] persisted to the recall visit, up to 2 years post treatment initiation.
As the study was not designed to assess the long-term efficacy of PLLA, treatment was limited to three sessions and many patients subsequently sought additional PLLA treatments. This could have been anticipated, as in the clinical setting it is likely that moderately to severely lipoatrophic patients, such as those enrolled here, would require up to five PLLA treatment sessions [10,11]. Nevertheless, analysis of results when patients who received additional treatments were excluded indicated that benefits derived from PLLA received during the study persisted to the recall visit.
The most common treatment-related AE at 2 years was the occurrence of small, palpable, but nonvisible nodules, the investigation of which was not planned for in the study protocol. Uneven dispersal of PLLA in the deep dermis can lead to localized overstimulation of the fibroblasts, manifesting as nodules or papules. These nodules were not physically obvious and no patient reported that they had sought treatment or described them as painful or troublesome. Furthermore, because only two of nine patients reported that the lumps were visible, it may be more accurate to describe these events as subcutaneous papules (<5 mm); however, histological examination is needed to define the nature of these lumps. The lumps were not associated with clinically evident inflammation [12-14], and no further investigation was clinically warranted or requested by the patients. Induration was defined as the general hardening or thickening of soft tissue areas and may represent movement of the PLLA from the deeper dermis into more superficial dermal planes. With the exception of subcutaneous papules, all of the AEs, including one case of infection and bruising, were reported at the time of injection. The one case of infection was a self-limiting superficial local cellulitis, which did not require antibiotic therapy (n=1) and was not cultured [8].
Although nodule formation is consistent with reports from other PLLA HIV lipoatrophy studies [7,15], the incidence here (31%) is higher than has been reported elsewhere. For example, two trials, each of which enrolled 99 patients with HIV-related lipoatrophy, reported the incidences of subcutaneous papules at 1 year to be 6 and 13% [15]. Another investigator reported the incidence of nodules to be much rarer (0.139%; one of 722 patients) in a diverse European population of patients seeking PLLA treatment for both age- and HIV-related lipoatrophy [16]. Improving injection technique may be responsible for the apparent decrease in nodule formation observed over time. Since our study, injection technique has been refined: for example, it is now generally recommended that patients are treated with a more dilute concentration of PLLA (5 mL fluid per vial vs the 3 mL used here). This more dilute concentration provides a controllable injection of the product, such that only light-to-moderate pressure on the plunger is required, and the increased volume helps distribute PLLA evenly. As uneven product distribution is thought to be a major contributory factor to disappointing results, this, combined with massage of the treated area, both immediately after injection and daily by the patient in the following weeks, is strongly recommended [10].
This study highlights the need for an accurate, holistic definition of lipoatrophy that can be applied to both HIV-infected and cosmetic patients. A definition and a facial lipoatrophy scale, which will be validated in the near future, are in preparation. To conclude, PLLA appears to be well tolerated for the correction of severe facial lipoatrophy. The most common delayed AE, injection-site nodules, were of no clinical and limited aesthetic significance; however, further investigation is warranted to establish the cause of such nodules.
Methods
Patients and study design
This was a randomized, open-label, comparative, single-centre study of injected PLLA in patients with HIV-related facial lipoatrophy. Patients who were HIV positive, and with physician and patient-assessed moderate-to-severe nasolabial fat pad loss, were enrolled in the study. The degree of facial lipoatrophy was classified as normal, mild, moderate and severe. Eligible patients had received no previous treatment for the correction of their HIV-associated lipoatrophy. As part of the original study design, eligible individuals (n=30) were randomized in a 1 : 1 fashion to receive immediate (n=15) or delayed (n=15) treatment. All patients received three sessions, separated by fortnightly intervals, of bilateral injections of PLLA into the deep dermis overlying the buccal fat pads. For each treatment session, 0.15 g of PLLA was reconstituted by the addition of 2 mL of sterile water for injection and 1 mL of 2% lidocaine to give a total volume of 3 mL. Up to 3 mL of reconstituted PLLA was injected into the treatment area. Patients in the delayed group commenced treatment 12 weeks after those in the immediate group. Injection techniques, and details of assessments undertaken at time-points other than the recall visit, are reported elsewhere [8].
Written, informed consent was obtained at screening and again at the recall visit, which was not part of the original protocol. The original protocol was approved by an independent ethics committee at the Chelsea and Westminster Hospital, London, UK.
Assessments
All patient-reported adverse events (AEs) were recorded at the recall visit. AEs were recorded by system organ class, Medical Dictionary for Regulatory Activities (MedDRA) term, intensity and relationship to treatment. The patient recorded the presence or absence of nodules following injection and no formal measurement was made of these small lumps. Long-term efficacy was assessed subjectively using a VAS and HADS scores [9]. Ultrasound measurements were not made at the recall visits.
The VAS was completed at day 1 (baseline) and at the recall visit. Patients were asked to record how thin, on a 10 cm scale, they perceived their face, arms, legs, abdomen and buttocks. The HADS was completed on the same occasions to record the emotional state of patients, to test the hypothesis that effective treatment could improve levels of anxiety and depression caused by facial lipoatrophy.
Statistical analysis
As this was a pilot study that was extended to include a long-term follow-up visit (the recall visit), a minimal sample size was not predetermined. Data were analysed using SAS statistical software (SAS Institute, Cary, NC, USA). Differences between the immediate and delayed groups were evaluated using both the two-sample t-test and the nonparametric Wilcoxon rank-sum test. Changes from baseline within groups were analysed using the paired t-test and P-values of < 0.05 were considered significant.
RESULTS
Patients
Twenty-seven of the 30 patients originally enrolled agreed to return for the recall visit (immediate group: n=13; delayed group: n=14). The majority of patients were male (93%) and described themselves as white (93%) or black (7%). The mean age of patients was 41 years. There were two female patients. Baseline demographics and scores were similar between groups [8].
Safety
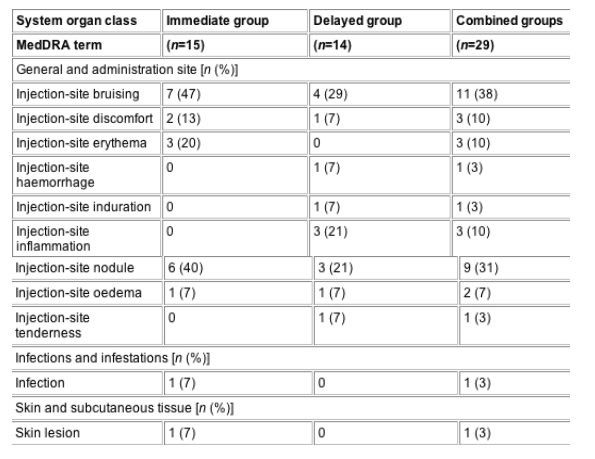
From baseline to the recall visit, a total of 112 treatment-emergent AEs were noted, which were reported to be mild, moderate and severe in intensity in 44, 47 and 7% of cases, respectively. Nine (60%) patients in the immediate group and eight (57%) in the delayed group experienced a total of 36 AEs thought to be related to the treatment during the study. None of these events were serious and all were localized to the injection site and cheek area (Table 1).
With the exception of nodules and induration, and one case of infection (noted 2 weeks after the first treatment session), all treatment-related AEs were reported at the time of injection. The single case of injection-site induration and all nine cases of injection-site nodules were noted at the recall visit. Exact dates of onset could not be provided by patients and no further histological examination of the sites was performed. All patients described nodules as 'lumps' or 'bumps' with the majority (56%; five of nine) of patients describing the lumps as either 'small' or 'slight'. All lumps were palpable but generally nonvisible: two patients reported that their lumps were occasionally visible.
Table 1 Incidence of treatment-related adverse events
Efficacy
The favourable trends in the efficacy results noted at week 24 [8] continued to the recall visit, as captured by subjective assessments of patients' satisfaction with their facial appearance and levels of anxiety and depression.
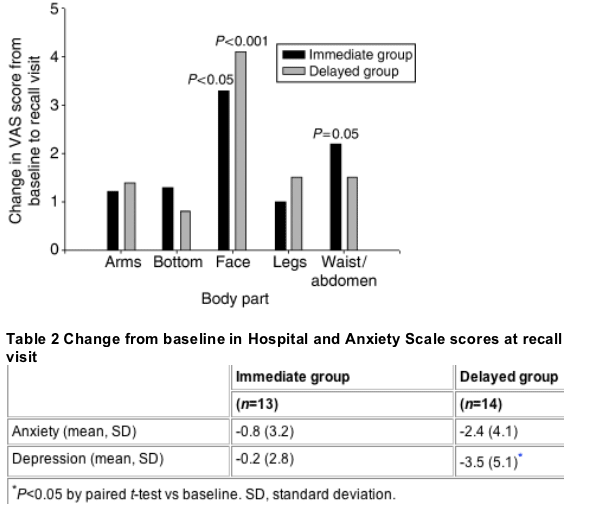
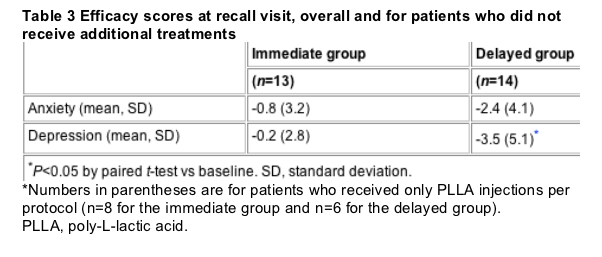
According to the VAS scores, patients' self-perceived facial thinness was significantly more positive at the recall visit than at baseline. In contrast, other areas of the body, with the exception of the waist/abdomen in the immediate group, did not show significant improvements (Fig. 1). According to the HADS, patients in both groups were less depressed and anxious at the recall visit than at baseline. However, these improvements only reached statistical significance for depression in the delayed group (P=0.029) (Table 2).
It should be noted that 14 of 27 patients at the recall visit reported that they had received additional PLLA treatments (range 1-10) between the end of the initial study and the recall visit. Of these 14 patients, one did not report how many additional treatments they had received and the recording of 17 further sessions for one patient is thought by the investigators to be an administrative error. The amount of product used and timings of additional sessions were not reported for all patients. Ten of the patients specified that they received injections to other areas of the face, most commonly the temporalis regions.
Analysis of VAS and HADS scores when patients who received additional treatments are excluded revealed that these supplementary treatments did not skew the overall efficacy results reported at the recall visit. In both the immediate and the delayed groups, trends for improved anxiety, depression and VAS scores from baseline persisted, with the exception of a modest increase in anxiety score in the immediate group. Furthermore, results were more positive when patients who had received additional treatment were excluded in the case of delayed-group anxiety and depression scores and immediate-group depression and VAS scores (Table 3).
Fig. 1 Improvements from baseline in visual analogue score (VAS) of patient-perceived thinness. Improvements for the waist/abdomen in the immediate group and in the face for both the immediate and delayed groups were statistically significant (P=0.05, P<0.05, P<0.001, respectively).
|
|
| |
| |
|
|
|