| |
Herpes Simplex: affect of HAART, HIV-RNA, CD4 Count
|
| |
| |
Herpes simplex virus infection in women in the WIHS: Epidemiology and effect of antiretroviral therapy on clinical manifestations
AIDS: Volume 20(7) 24 April 2006 p 1051-1058
[EPIDEMIOLOGY AND SOCIAL]
".....In the WIHS women, increases in CD4 cell counts and control of HIV viremia were associated with fewer genital ulcers on examination, perhaps due to a diminution in the duration of recurrences, but had minimal effects on the occurrence of genital sores. This finding is consistent with the recent report, which found that HAART treatment did not significantly reduce the frequency of HSV reactivation in HIV-infected persons. However, HAART was associated with fewer days with a HSV lesion. Our findings indicate that HAART-induced improvements in immune function and control of HIV viremia has a beneficial effect on recurrences of genital herpes in HIV infected women, but not enough to eliminate all the effects of HIV infection on HSV. Higher rates of genital lesion among HIV-infected women could lead to a greater risk of HSV-2 and HIV-1 transmission. Thus, anti-HSV therapy might have additional benefit in preventing transmission of both HSV-2 and HIV-1 infection....."
authors: Ameli, Niloufara; Bacchetti, Petera; Morrow, Rhoda Ashleyb; Hessol, Nancy Aa; Wilkin, Timothyc; Young, Maryd; Cohen, Mardgee; Minkoff, Howardf; Gange, Stephen Jg; Greenblatt, Ruth Ma
From the aUniversity of California, San Francisco School of Medicine, San Francisco, California, USA
bUniversity of Washington, School of Medicine, Seattle, Washington, USA
cWeill Medical College of Cornell University, New York, New York, USA
dGeorgetown University School of Medicine, Washington DC, USA
eCook County Medical Center, Chicago, Illinois, USA
fState University of New York at Downstate, School of Medicine, New York, New York, USA
gJohns Hopkins University School of Public Health, Baltimore, Maryland, USA.
Abstract
Objective: To determine the prevalence of infection with herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) among women with and at high risk for HIV infection, and to evaluate the effect of HAART on the recurrence of genital lesions.
Methods: We evaluated the epidemiology and clinical manifestations associated with HSV-1 and HSV-2 among 1796 HIV-infected and 476 HIV-uninfected women enrolled in a multisite cohort study. Serum antibodies to HSV-1 and HSV-2 at baseline and self-reported history of genital herpes, reports of recent genital sores and presence of genital ulcers on examination, and use of HAART regimen at each study visit were analyzed.
Results: Reactivity to HSV-1 only and HSV-2 only was detected in 18% and 20% of HIV-infected, and in 28% and 18% of HIV-uninfected participants respectively; 58% of HIV-infected women and 45% of HIV-uninfected women were seropositive for both HSV types. Reactivity to HSV-2 was associated with increasing age, more male sexual partners, earlier sexual debut, African-American race, Latina ethnicity, less education and lower income. HIV-uninfected women reported significantly fewer genital sores than HIV-infected women who had used HAART for at least 1 year and had optimal CD4 cell gain and viral suppression (adjusted odds ratio (OR), 0.19; 95% confidence interval (CI), 0.13-0.28).
Conclusion: Use of HAART and subsequent immune recovery does not completely eliminate the effect of HIV infection on genital lesions among women with concurrent HSV-2 infection.
Introduction
Herpes simplex virus (HSV) infections are endemic worldwide [1-3]. Infections with HSV-2 typically affect anogenital sites and are transmitted sexually or acquired at birth [4-6]. In contrast, HSV-1 commonly causes orolabial infection, and transmission most frequently occurs via non-genital personal contact [7]. Both viruses are capable of causing ulcerative infections of the oral and genital mucosa, and can produce recurrent lesions that are influenced by host immune response [8].
HIV-1 infection is associated with genital HSV infection [9-12]. HSV probably enhances the transmission and acquisition of HIV through disruption of the genital epithelium and persons with genital ulcers are at higher risk for acquiring HIV than persons without ulcers [13-15]. HSV-2 is a significant opportunistic infection in HIV-infected persons, often resulting in frequent reactivation and severe, long lasting or recurrent genital lesions [16,17].
Since the mid-1990s, HAART has been shown to be effective in prolonging survival and slowing the progression to clinical AIDS in HIV-infected individuals [18-23]. In North America women comprise a substantial and increasing proportion of new HIV diagnoses and new AIDS cases. While few studies have investigated the prevalence and risk factors for HSV infections in women with HIV, little is known about how the clinical manifestations of HSV among HIV-infected women have changed since the advent of HAART.
We examined the epidemiology of HSV-1 and -2 among HIV-infected and -uninfected women at the time of their enrollment into a longitudinal cohort study, and determined the effects of HAART on the occurrence of genital lesions among women with pre-existing infections with HSV-1 and/or HSV-2.
RESULTS
Factors associated with genital lesions among HIV-infected women before and after HAART
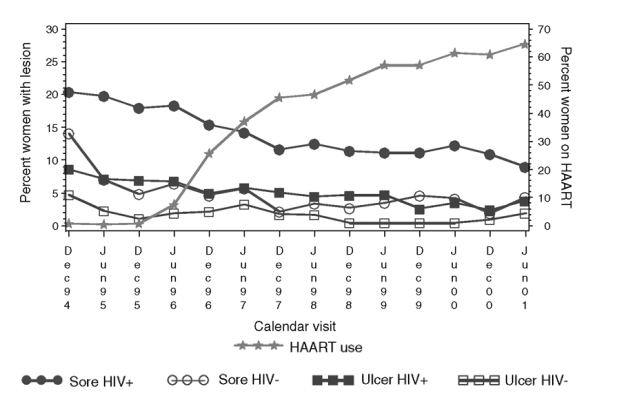
The results of the random effects logistic regression models constructed to evaluate the effect of HAART on report of genital sore and presence of genital ulcer on examination are summarized in Table 4. The women with HSV-1 only infection were less likely to have genital ulcer on examination (adjusted OR, 0.35; 95% CI, 0.22-0.58) or to self-report a genital sore (adjusted OR, 0.28; 95% CI, 0.18-0.43) than women with HSV-2 only or both infections. However, no statistically significant differences in the risk of genital lesions, upon examination or self-report, were found between women with HSV-2 only and dual type infection. Per each decade of age, older women were less likely than young women to have genital ulcer on examination (adjusted OR, 0.82; 95% CI, 0.70-0.97) or to report a genital sore (adjusted OR, 0.73; 95% CI, 0.62-0.87).
In models that controlled for plasma HIV RNA and CD4 cell counts, HAART did not substantially reduce the occurrence of genital lesions in HIV-infected women (either at the first or subsequent HAART visits) compared with pre-HAART visits. Greater plasma HIV RNA levels were associated with a higher risk (adjusted OR, 1.41; 95% CI, 1.13-1.76) to have had an ulcer identified during examination. Self-report of genital sore did not appear to be substantially influenced by HIV RNA level in this model.
Women with recent CD4 cell counts of < 350 cells/ml were 1.35-2.15 times more likely to have genital ulcer on examination than women with higher CD4 cell counts. Furthermore, women with mid-range recent CD4 cell counts (200-349 cells/ml) had a 40% increased risk of genital ulcer on examination if their nadir CD4 cell count was less than 200 cells/ml (adjusted OR, 1.90; 95% CI, 1.25-2.90) when compared to women whose CD4 cell count had never fallen below 200 cells/ml during study observation (adjusted OR, 1.35; 95% CI, 0.96-1.90).
We also examined the effects of elapsed time between study visits. Women with a longer time between study visits had an increased risk of reporting a genital sore since the last visit (adjusted OR, 2.03/year; 95% CI, 1.08-3.79). The model also demonstrates a significant decrease over time in self-report of genital sore (adjusted OR, 0.85; 95% CI, 0.80-0.91), and a non-significant decrease in genital ulcer on examination.
To evaluate the effect of HSV medication on occurrence of genital lesion, we used two approaches. First, we added HSV medication as a dichotomous predictor to the random effects logistic regression models. The self-report of genital sore was significantly higher among HIV-infected women who reported using HSV medication as compared to HIV-infected women who did not (adjusted OR, 1.91; 95% CI, 1.52-2.39). This likely reflects women using HSV medication to treat herpes outbreaks rather than to prevent them. The occurrence of genital ulcer on examination was not significantly associated with the use of HSV medication (adjusted OR, 1.13; 95% CI, 0.82-1.56). In the second approach, we performed the random effects logistic regression analysis both excluding and including study visits on HSV medication. We observed no significant difference in the parameter estimates, adjusted OR, and 95% CI between the two models. The results shown in Table 4 changed little when HSV medication was added to the models or when visits were excluded from the analyses if the participant reported using HSV medications.
Differences between HAART recipients and HIV-uninfected women
To further evaluate the effect of HAART on genital lesions in women seropositive for single or dual HSV types, we compared the prevalence of genital lesions in HIV-uninfected women and HIV-infected women with undetectable HIV RNA (< 4000 copies/ml), CD4 ≥ 350 cells/ml, and who reported taking a HAART regimen for at least 1 year. The self-report of genital sore was significantly less frequent among HIV-uninfected women compared with HIV-infected HAART responders (adjusted OR, 0.19; 95% CI, 0.13-0.28). The occurrence of genital ulcer on examination was also lower, but not statistically significantly so, among HIV-uninfected women compared with HAART responders (adjusted OR, 0.68; 95% CI, 0.44-1.05). Genital ulcers were identified on exam significantly less frequently over time among HIV uninfected women (adjusted OR, 0.80; 95% CI, 0.70-0.92) but this decrease appeared to be much weaker among HAART responders (adjusted OR, 0.94; 95% CI, 0.86-1.03). In contrast self-reported genital sores decreased substantially over time among both the HAART responders (adjusted OR, 0.85; 95% CI, 0.79-0.90) and the HIV-uninfected women (adjusted OR, 0.80; 95% CI, 0.73-0.88). We also estimated and tested the interaction of HIV status and decrease in self-report of genital sores over time (adjusted OR, 1.06; 95% CI, 0.95-1.18).
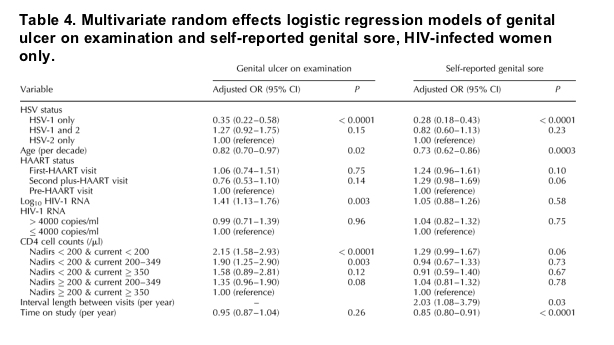
The self-report of genital sore and occurrence of ulcer on examination are presented in Fig. 1, which shows a decrease over time in the percent of women with genital lesions, in both HIV-infected and HIV uninfected women.
Fig. 1. Occurrence of genital lesion and HAART use among HSV-2 seropositive HIV-infected and HIV uninfected women. Self-report of genital sore, finding of genital ulcer on examination, and use of HAART over time in the WIHS. Study visits occurring between October 1 and March 31 are categorized into 'Dec' group category. Study visits occurring between April 1 and September 30 are categorized into 'Jun' group category.
Demographic and behavioral characteristics of HSV-1 and HSV-2 seropositive women
A total of 1796 HIV-infected women and 476 HIV-uninfected women were included in this analysis. The HIV-infected and uninfected participants were similar with respect to age, race/ethnicity, level of education, and per capita household income. Eighteen percent of HIV-infected women were seropositive for HSV-1 only, 20% for HSV-2 only, and 58% had antibodies to both HSV types (Table 1). HIV-uninfected women were more likely to have antibodies to HSV-1 only (P < 0.01) and less likely to have antibodies to HSV-2 than HIV-infected women (P < 0.01). The prevalence of antibodies to HSV-1 only was the highest in the 17-29 year-old-group (28%). By contrast, HSV-2 only and dual type infections were most prevalent in the 40-49-year-old-group (22% and 62% respectively).
Serologic reactivity to HSV varied by ethnicity. White women were significantly more likely to be seronegative than persons of other ethnicity (P < 0.01). Latina/Hispanic women had the highest prevalence of HSV-1 only. For HSV type 2 infection, African-American and Latina/Hispanic women had lower seroprevalence of HSV-2 only infection (P < 0.01), but were more likely to have dual type infection (P < 0.01). The prevalence of dual type infections was higher in persons who had first sexual contact at a younger age (P < 0.01) and increased with the total number of lifetime male partners. The seroprevalence of HSV-2 only infection showed a similar association with these two behavioral factors.
Clinical characteristics of HSV-1 and HSV-2 seropositive women
At study enrollment, a history of genital herpes was reported by 15 (13%) of women who had no antibodies to either HSV type, 49 (11%) of the HSV-1 only infected women, 133 (30%) of the HSV-2 only infected women, and 279 (22%) of the women with antibodies to both HSV types reported a history of genital herpes (Table 2). Self-report of genital sore and the presence of a genital ulcer on examination were associated with HSV serologic reactivity. Furthermore, of the 351 women who self-reported a genital sore at the baseline visit, 87% were HSV-2 seropositive. Similarly, of the 136 women who had genital ulcers detected upon examination, 90% were seropositive for HSV 2.
The sensitivity and specificity of self-reported history of genital herpes for HSV-2 seropositivity varied by HIV status. HIV-infected women had higher sensitivity than HIV-uninfected women (0.27 versus 0.09). In contrast specificity was higher for HIV-uninfected compared to HIV-infected women (0.96 versus 0.86).
Factors associated with serologic reactivity to HSV types 1 and 2 at enrolment
The results of multivariate logistic regression analyses of factors associated with serologic reactivity to HSV-1 or HSV-2 are summarized in Table 3. Reactivity to both HSV types was associated with African-American and Latina/Hispanic ethnicity (compared with White) and having a household income less than $18 000. The presence of HSV-1 antibodies was associated with lesser maternal education [adjusted odds ratio (OR), 1.68; 95% confidence; (CI), 1.29-2.19], but did not appear to be strongly associated with number of sexual partners or age.
The presence of antibody to HSV-2 was associated with HIV coinfection (adjusted OR, 1.96; 95% CI, 1.48-2.59), increasing age (adjusted OR, 1.77; 95% CI, 1.49-20.9), less than 12 years' education (adjusted OR, 1.36; 95% CI, 1.00-1.84), and being 17 years of age or younger at the time of first sexual intercourse (adjusted OR, 1.38; 95% CI, 1.03-1.85). We also observed a strong concordance between increasing number of lifetime male sexual partners and the prevalence of HSV-2 antibodies.
Discussion
In the WIHS, HSV infections were highly prevalent in both the HIV-infected (96%) and HIV-uninfected (92%) individuals. Dual type infections were present in the majority of HIV-infected women, and almost half of HIV-uninfected women. The prevalence of type 2 infections was higher than that reported for many other populations [1,30] in both the HIV-infected and HIV-uninfected groups. The finding of type 2 infection in association with increasing age, African-American race, lesser educational attainment and lower income is consistent with previous reports [1,30]. Higher rates of HSV-2 infection are reported in persons with HIV infection [31,32] and patients attending sexually transmitted diseases clinics [30] and HIV test sites [31]. Also consistent with previous reports, the majority of the women who had serologic evidence of HSV-2 infection failed to report a history of genital herpes. HSV-1 infections were associated with lower income and lesser maternal educational attainment, which are both indicators of socioeconomic status. Serologic reactivity to HSV-1 increased at a younger age than reactivity to type 2 and was also more evenly distributed by ethnicity.
Our data indicate that HIV-infected women with the most favorable values for HIV-specific predictors such as HIV RNA < 4000 copies/ml, CD4 count ≥ 350 cells/ml, and at least 1 year HAART use were still at significantly higher risk to self-report genital sores than HIV-uninfected women. We also observed a trend toward greater occurrence of genital ulcer on examination among HAART treated HIV-infected women compared to HIV-uninfected women.
Two measures of genital herpes lesion occurrence were used for this report. One measure was self-report of genital sore during the interval between the twice annual study visits; self-reported genital sore was associated with HSV-2 serologic reactivity, the presence of HIV infection and lack of CD4 cell count increase from nadir to above 200 cells/ml after severe depletion during HAART. Recollection of genital sores may not be accurate, and events may either be over- or under-reported depending on knowledge of the diagnosis and severity of the symptoms. Nonetheless, it does present an indicator of how noticeable herpetic recurrences were for the study participant.
The other measure of herpes genital lesion occurrence obtained in this study was identification of genital ulceration during pelvic examination. Because WIHS visits occurred twice a year, and were not scheduled to coincide with symptoms or clinical events, these data are based on a random evaluation for the presence of ulcers. The chance that an ulcer would be present during a WIHS visit is increased with frequent reactivation and duration of the lesion. Duration of herpetic lesions is associated with immune competence; chronic ulcers of > 1-month duration are an AIDS defining condition. The presence of genital ulcerations on examination was associated with type 2 and dual type infection, higher viral loads and most strongly associated with extent of CD4 cell depletion and recovery on HAART, particularly increases from cell counts of less than 200 to 200-349 cells/ml.
A few other limitations of this study deserve mention. Our cohort consisted only of women; therefore the results of this study may not be generalized to HIV-infected men. Only sera obtained at enrollment were tested for HSV type-specific antibodies; therefore participants who seroconverted for HSV-1 and/or HSV-2 during the 7-year follow up are misclassified in the random effects logistic regression model. This study was not able to assess the frequency of HSV reactivation or severity of the genital lesion with respect to HAART use. HAART may change the course of HSV infection, despite no significant change in occurrence of genital lesion.
In the WIHS women, increases in CD4 cell counts and control of HIV viremia were associated with fewer genital ulcers on examination, perhaps due to a diminution in the duration of recurrences, but had minimal effects on the occurrence of genital sores. This finding is consistent with the recent report, which found that HAART treatment did not significantly reduce the frequency of HSV reactivation in HIV-infected persons. However, HAART was associated with fewer days with a HSV lesion [33]. Our findings indicate that HAART-induced improvements in immune function and control of HIV viremia has a beneficial effect on recurrences of genital herpes in HIV infected women, but not enough to eliminate all the effects of HIV infection on HSV. Higher rates of genital lesion among HIV-infected women could lead to a greater risk of HSV-2 and HIV-1 transmission. Thus, anti-HSV therapy might have additional benefit in preventing transmission of both HSV-2 and HIV-1 infection.
Methods
Study population and data collection
The Women's Interagency HIV Study (WIHS) is a prospective study of HIV-1 infection in women, conducted in five locations within the USA: New York City (two sites), the Washington DC area, Chicago, Southern California, Hawaii and the San Francisco Bay area. The WIHS methods and baseline cohort characteristics have been described previously [24]. Briefly, from October 1994 through November 1995, 2628 women (2059 HIV-1 seropositive and 569 seronegative) were enrolled in the WIHS. The HIV-infected and -uninfected women were recruited from similar sources and were matched for demographic and risk factors for acquisition of HIV infection.
WIHS participants were interviewed every 6 months, underwent physical and gynecological examinations and provided blood specimens. In this study, history of genital herpes and genital sores were ascertained through self-report. To ascertain history of genital herpes, the participant was asked at the baseline study visit if a health care provider has ever told her that she had herpes in or around her genital area. To ascertain report of genital sores, the participant was asked at each study visit if she has experienced a sore or ulcer in or around her genital area since her last study visit. Trained research clinicians detected genital ulceration during pelvic examinations at the semi-annual study visits. HAART was defined according to the 2004 Department of Health and Human Services guidelines [25].
For this report, 356 (13.5%) women were excluded from the analyses [HIV seroconversion after study enrollment (n = 11; 0.5%), missing or indeterminate HSV serology results (n = 133; 5%), missing number of lifetime male sexual partners, household income, or years of education (n = 212, 8%)], leaving 2272 participants for the cross-sectional analysis. To evaluate the prevalence of genital lesions before and during use of HAART and to correlate these findings with immunologic and virologic data, we further restricted the sample in the longitudinal analyses to HIV-infected women who had reported initiating HAART and for whom HIV RNA levels and lymphocyte subset measurements were available (n = 1581; 1105 HAART initiators, 476 HIV-uninfected). Observations from up to 14 study visits per woman over a time period of 7 years (1 October 1994-30 September 2001) were included in these analyses. This provided 13 606 complete visits, with over half of the women contributing nine or more. During the study period, of the 2272 women included in the analyses, 182 (134 HIV-infected, 48 HIV-uninfected) dropped out and 463 (444 HIV-infected, 19 HIV-uninfected) died.
Laboratory methods
Sera obtained at enrollment were tested for HSV type-specific antibodies using a commercially available type 1 and type 2 glycoprotein G-based enzyme immunoassay (gG-EIA) (Gull Laboratories, Salt Lake City, Utah, USA), as described previously [26]. Sera with negative or equivocal results in either gG1- or gG2-EIA were tested by Western blot as previously described [27,28]. The sensitivity of the gG-EIA for HSV-1 is 95% and the specificity is 96%. For HSV-2, the sensitivity and specificity are 98% and 97%, respectively [26].
Quantification of HIV RNA in plasma was performed using the isothermal nucleic acid sequence based amplification (Nuclisens) method (bioMerieux, Durham, North Carolina, USA). Lymphocyte subsets were quantified using standard flow cytometric methods.
Statistical analysis
Serologic reactivity to HSV-1 and HSV-2 measured at enrollment, self-reports of genital sores, and observations of genital ulcers on examination measured at each study visit were each analyzed as dichotomous outcome variables. Multivariate logistic regression models were used to evaluate the associations between HSV-1 and HSV-2 serologic reactivity and demographic and behavioral variables.
The temporal trends in the prevalence of self-reported genital sore and finding of ulcer on examination were evaluated using repeated measures, random effects logistic regression models of multiple visits per woman, with predictors that could differ for the same woman at different visits. A random subject effect was included to account for the dependence of multiple observations from individual participants [29]. The primary predictors of interest were use and duration of HAART. We also examined age (in decades), interval length between study visits (per year), time on study (per year), plasma HIV RNA, and nadir and current CD4 cell count.
To evaluate the effect of HAART on self-reported genital sores and genital ulcer on examination, we limited the analysis to data from HIV-infected women who reported using HAART at one or more WIHS visits. We defined three categories of study visits [pre-HAART (reference), first HAART, and second plus HAART], and these could change within each woman over different study visits. The pre-HAART category was defined as study visits prior to HAART initiation. The first HAART category was defined as the first study visit during which HAART use was reported. The second plus HAART category was defined as all study visits on-HAART after the first HAART study visit.
We used two approaches to define first HAART and second plus HAART categories. First, we performed the random effects logistic regression using only study visits with consecutive HAART use after HAART initiation. This excluded all study visits after the first gap in HAART from the analysis. In the second approach, women with non-HAART study visits after HAART initiation had those non-HAART visits categorized back into the pre-HAART group. At the subsequent visits, if HAART was reported, the visit was categorized into first or second plus HAART categories. The two approaches yielded similar findings; therefore we present results using the first approach.
To study the effect of plasma HIV RNA on the prevalence of genital lesions, we used log10 HIV RNA as a time-varying predictor. In addition, we examined whether having a higher than the limit of plasma HIV RNA quantification (4000 copies/ml) was associated with increased risk of genital lesions. To investigate the effect of changes in CD4 lymphocyte counts on self-reported genital sores and presence of genital ulcer on examination, we used five categories of CD4 nadir and current CD4 cell counts measured at the study visits (nadir < 200 and CD4 < 200, nadir < 200 and CD4 200-349, nadir < 200 and CD4 > 349, nadir ≥ 200 and CD4 200-349, and nadir ≥ 200 and CD4 > 349 cells/ml (reference)]. Current CD4 cell count is defined as the CD4 measured at the study visit. CD4 nadir is defined as the lowest CD4 cell count up to or including the visit.
We performed similar repeated measures, random effects logistic regression models including both HIV-infected and uninfected women, with HIV positivity included as an additional predictor. In these models, we compared the HIV-infected women having the most favorable values for HIV-specific predictors at all visits, to the HIV-uninfected women, so that the estimated HIV positivity effect would quantify the odds ratio comparing HIV-infected women with HIV RNA < 4000 copies/ml, nadir CD4 cell count > 200/ml, current CD4 cell count > 350/ml, and HAART for two or more visits to HIV-uninfected women with the same HSV serology, age, and time on study.
Statistical analyses were performed using SAS software version 8.2 (SAS Institute, Cary, North Carolina, USA).
|
|
| |
| |
|
|
|