| |
HAART Increased AIDS Survival At least 13 Years; HIV Testing Should Routinized
|
| |
| |
The Journal of Infectious Diseases July 1, 2006;194:11-19
...Projected per-person survival after an AIDS diagnosis increased from 19 months (1.6 years) in the absence of treatment to 179 months (14.9 years) by 2003, a gain of 160 months (13.3 years)...... analysis was conservative; when assumptions were necessary, they were designed to underestimate the total survival benefit. For example, we limited the size of the eligible patient population to those with AIDS... improvement in AIDS-associated survival in the United States over time. Median survival was 1.7 years for the PCP era, 7.5 years for ART 1, and 14.1 years for ART 4.
.... our findings not only demonstrate the striking survival gains achieved via advances in AIDS treatment but also emphasize the importance of expanded HIV testing and linkage to care, so that greater numbers of infected persons can access lifesaving therapy.... Developing drugs, testing them without undue delay, accelerating their regulatory approval, and making them widely available have saved lives... Further investment in outpatient care should emphasize voluntary counseling and testing programs for HIV diagnosis that are integrated into routine medical care... Further investment in outpatient care should emphasize voluntary counseling and testing programs for HIV diagnosis that are integrated into routine medical care...
... offering and encouraging testing as a routine part of medical screening. We must reduce barriers to care, the first of which is the difficulty with which a test is obtained in many venues. We do not require extensive, expensive, time-consuming, and intimidating pretest counseling before screening for diabetes, for example, another disease that is lethal if unmanaged but that is controllable with lifelong medication. Yet many US guidelines demand substantial counseling infrastructures that may discourage primary-care providers from offering HIV tests as easily as they can offer a urine dipstick for glucose to screen for diabetes. Posttest counseling is essential for psychosocial assistance, a bridge to HIV-related health care, and needed to reduce high-risk behaviors, but pretest counseling can be made more efficient to reduce at least one barrier of time and money...
Walensky et al. highlight the importance of detecting all persons infected and providing care to all those who know their HIV status. Innovation is needed on many fronts....Brief health education messages that are designed for clinicians to deliver within the care setting have assisted persons in HIV care to reduce their high-risk behaviors. Practitioners in lower-prevalence regions suggest minimizing pretest counseling through interview-based risk triage, reserving their staff time for the essential posttest counseling sessions. Rapid tests are used widely in developing-world settings .....Rapid tests are an innovation used far less often in the United States than they should be....
"As new HIV therapies have come into the clinic, we have witnessed the transformation of HIV/AIDS from a rapidly fatal disease into a controllable condition," notes NIAID Director Anthony S. Fauci, M.D.
A lethal disease has been transformed into a chronic, manageable condition wherever health services delivery, financing, drug logistics (especially critical in rural areas and developing countries), health manpower, health policy, and health psychology are applied successfully.....
The researchers used a computer model, developed by Dr. Freedberg and colleagues, that incorporates literature-based data of clinical measures including HIV viral load, CD4+ T-cell counts (a measure of immune system health), efficacy of HAART, and incidence of opportunistic infections, to simulate HIV disease progression both with and without treatment. Information about the number of people diagnosed with AIDS and accessing health care each year between 1989 and 2003 came from U.S. Centers for Disease Control and Prevention surveillance and other published data.
"....The model of Walensky et al. gives all of us, from our complementary disciplines, cause for celebration.... This latter-20th-century advance in antiviral therapy has its centennial parallel in the golden era of microbiology and vaccine and drug development in the late 19th and early 20th centuries.... 21st century grounding in molecular methods augurs well for future discoveries leading to an eventual cure for HIV infection, flushing out and killing virus that is latent in deep tissues. This may be a good time for our national political leaders to reconsider their decision to slow the growth of the budget of the US National Institutes of Health, now lagging behind the rate of inflation.... Use of ART to block HIV transmission from mother to infant has virtually eliminated pediatric HIV infection as a major public-health problem in the United States and other economically prosperous nations. Easier-to-implement nevirapine and nevirapine-zidovudine regimens were developed that could be applied anywhere in the world..." From EDITORIAL
The investigators defined six eras of AIDS treatment between 1989 and 2003. In the first two periods, 1989 to 1992 and 1993 to 1995, drugs became available to prevent two common infections--Pneumocyctis jirovecii pneumonia and Mycobacterium avium complex.
"This type of research can also be used to understand the tremendous survival benefits that can be gained globally by continued rapid expansion of access to these very effective HIV/AIDS treatments in resource-limited settings," adds Dr. Freedberg. "This expansion is of critical importance."
EDITORIAL
Millions of Life-Years Saved with Potent Antiretroviral Drugs in the United States: A Celebration, with Challenges
Sten H. Vermund
Institute of Global Health and Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee
In this issue of the Journal, Walensky et al. estimate the benefits that have been gained from multidrug antiretroviral therapies (ARTs) since 1989 [1]. Their finding of about 3 million years of life saved in the United States quantifies ART benefits at the population level, complementing the well-known data on plummeting US death rates and lower AIDS case report rates noted in the era of potent therapy [2, 3]. The authors' detailed sensitivity analyses, varying key estimated parameters in their models, indicate that less-conservative assumptions generate an estimate of >5 million years of life saved, a plausible "higher-end" estimate of benefit. The typical HIV-infected person now receiving potent combination ART lives at least 13-14 years longer than if he or she were to forego this therapy or if it were otherwise unavailable [1]. Quantifying the survival benefits of expanded diagnosis and modern care suggests that the economic and humanitarian benefits are greater than were hitherto appreciated.
The millions of life-years saved in the United States should reinvigorate policy debates as to how best to identify HIV-infected persons in our country by offering and encouraging testing as a routine part of medical screening. We must reduce barriers to care, the first of which is the difficulty with which a test is obtained in many venues. We do not require extensive, expensive, time-consuming, and intimidating pretest counseling before screening for diabetes, for example, another disease that is lethal if unmanaged but that is controllable with lifelong medication. Yet many US guidelines demand substantial counseling infrastructures that may discourage primary-care providers from offering HIV tests as easily as they can offer a urine dipstick for glucose to screen for diabetes. Posttest counseling is essential for psychosocial assistance, a bridge to HIV-related health care, and needed to reduce high-risk behaviors, but pretest counseling can be made more efficient to reduce at least one barrier of time and money. See full Editorial at end of this report.
The Survival Benefits of AIDS Treatment in the United States
The Journal of Infectious Diseases July 1, 2006;194:11-19
"....Projected per-person survival after an AIDS diagnosis increased from 19 months (1.6 years) in the absence of treatment to 179 months (14.9 years) by 2003, a gain of 160 months (13.3 years)...... the current magnitude of the life-expectancy gain from AIDS treatment is largely attributable to combination ART; this has transformed AIDS from a fatal disease to a highly treatable chronic condition, with average survival from AIDS diagnosis that is now >14 years. .......This survival benefit greatly exceeds that achieved for patients with many other chronic diseases in the United States....As many as 312,000 of the estimated 1,185,000 people infected with HIV in the United States are thought to be unaware of their serostatus....Of those who are aware of their infection, only 57% are estimated to be in care.....our findings not only demonstrate the striking survival gains achieved via advances in AIDS treatment but also emphasize the importance of expanded HIV testing and linkage to care, so that greater numbers of infected persons can access lifesaving therapy....."
Authors: Rochelle P. Walensky,1,2,5 A. David Paltiel,6 Elena Losina,4 Lauren M. Mercincavage,1 Bruce R. Schackman,7 Paul E. Sax,5 Milton C. Weinstein,3 and Kenneth A. Freedberg1,2,3,4
1Divisions of Infectious Disease and General Medicine, Department of Medicine, Massachusetts General Hospital, and 2Center for AIDS Research, Harvard Medical School, and 3Department of Health Policy and Management, Harvard School of Public Health, 4Departments of Biostatistics and Epidemiology, Boston University School of Public Health, and 5Division of Infectious Disease, Brigham and Women's Hospital, Boston; 6Division of Health Policy and Administration, Yale School of Medicine, New Haven, Connecticut; 7Department of Public Health, Weill Medical College of Cornell University, New York, New York
(See full editorial commentary by Vermund at end of report)
This analysis was conservative; when assumptions were necessary, they were designed to underestimate the total survival benefit. For example, we limited the size of the eligible patient population to those with AIDS; we excluded the early benefits of antiretroviral mono- and dual-drug therapy when survival benefits were more limited and HIV RNA data were not available; we utilized lower estimates for rates of linkage to care; we assumed that those who did not access care in the first year of their AIDS diagnosis were never eligible; and we omitted any benefits of reduced HIV transmission from care. Sensitivity analyses were performed to examine the stability of the results in the face of alternative assumptions regarding delays in the adoption of clinical guidelines, mean CD4 cell count at presentation, the number of patients entering care, and ART efficacies.
ABSTRACT
Background. As widespread adoption of potent combination antiretroviral therapy (ART) reaches its tenth year, our objective was to quantify the cumulative survival benefits of acquired immunodeficiency syndrome (AIDS) care in the United States.
Methods. We defined eras corresponding to advances in standards of human immunodeficiency virus (HIV) disease care, including opportunistic infection prophylaxis, treatment with ART, and the prevention of mother-to-child transmission (pMTCT) of HIV. Per-person survival benefits for each era were determined using a mathematical simulation model. Published estimates provided the number of adult patients with new diagnoses of AIDS who were receiving care in the United States from 1989 to 2003.
Results.
Compared with survival associated with untreated HIV disease, per-person survival increased 0.26 years with Pneumocystis jiroveci pneumonia prophylaxis alone.
Four eras of increasingly effective ART in addition to prophylaxis resulted in per-person survival increases of 7.81, 11.05, 11.57, and 13.33 years, compared with the absence of treatment.
Treatment for patients with AIDS in care in the United States since 1989 yielded a total survival benefit of 2.8 million years.
pMTCT averted nearly 2900 infant infections, equivalent to 137,000 additional years of survival benefit.
Conclusions. At least 3.0 million years of life have been saved in the United States as a direct result of care of patients with AIDS, highlighting the significant advances made in HIV disease treatment.
Background
In the face of the global AIDS pandemic, advancement in the treatment of HIV infection has been striking, but this progress has been associated with substantial economic costs. In 2006, US federal governmental agencies will allocate $21 billion to HIV/AIDS activities [1]. This includes about $3 billion for HIV/AIDS research at the National Institutes of Health; $12.6 billion for treatment under Medicare and Medicaid and for Ryan White Care Act funds; $2.7 billion for global contributions; nearly $2 billion for cash and housing assistance; and almost $1 billion for prevention activities at the Centers for Disease Control and Prevention (CDC) and local health departments [1].
On 7 December 1995, the first protease inhibitor (PI), saquinavir, was approved by the US Food and Drug Administration, leading to the advent of highly active antiretroviral therapy (ART) for HIV disease. In light of the 10-year anniversary of this important breakthrough in HIV care, we sought to measure what 2 decades of medical research, patient care, and financial investment have produced in terms of overall survival gains. We employed a model-based approach, conducting repeated analyses to explore the clinical consequences of alternative patient-care-innovation pathways. Our objective was to quantify clinical progress in AIDS care, in terms of years of life saved as a result of advances in HIV therapies in the United States.
RESULTS
Per-person HIV disease treatment survival benefits among those receiving care. Results from the era of PCP prophylaxis alone show that mean per-person life expectancy increased by 3.1 months, compared with that in the absence of prophylaxis (table 2). In this era, 33% of patients survived to 1993 (the PCP and MAC disease prophylaxis era), but only 2% survived to 1996 to receive any highly active ART. Although the life expectancy benefit anticipated from PCP and MAC disease prophylaxis alone was just 2.6 months, the era of PCP and MAC disease prophylaxis led to a mean survival increase of 24.4 months. This increase is largely a result of 39% of this cohort surviving to 1996 and then benefiting from ART 1. PCP and MAC disease prophylaxis combined with ART 1, 2, and 3 resulted in mean per-person survival increases of 93.7, 132.6, and 138.8 months, respectively. By ART 4 (2003), comprehensive AIDS care resulted in a projected per-person survival gain of 159.9 months, or 13.3 years.
HIV disease treatment survival benefits. The number of patients entering care in the United States ranged from a maximum of 75,486 per year in the PCP and MAC disease prophylaxis era (1993-1995) to a minimum of 24,780 in ART 4 (2003). The total survival benefit ranged from 40,912 years in the PCP prophylaxis era to 832,179 years in ART 3. The cumulative survival benefit for AIDS-related OI prophylaxis and combination ART was 2,813,892 years. Of these, 1,184,851 years have already been realized, and 1,629,041 years are being accrued by current patients with AIDS in care. Model-based survival curves for patients who received diagnoses in the first year of each of the 6 treatment eras (1989, 1993, 1996, 1998, 2000, and 2003) illustrate the improvement in AIDS-associated survival in the United States over time (figure 2). Median survival was 1.7 years for the PCP era, 7.5 years for ART 1, and 14.1 years for ART 4.
pMTCT survival benefits. pMTCT produced notable survival benefits as well. In the zidovudine-alone era (1994-1999), perinatal zidovudine treatment averted 1056 infant infections (table 2). After 2000, when combination ART became widely used in pregnant women, 1839 infant infections were averted. Mean per-child survival gains for the averted infections ranged from 60.5 years if the child was born before 1996 (before combination ART) to 45.8 years during 1996-1999, when combination ART was available. pMTCT led to a survival benefit of 137,479 years (table 2).
The cumulative survival benefit of AIDS-related OI prophylaxis, combination ART, and pMTCT in the United States was 2,951,371 years.
Sensitivity analyses. Several sensitivity analyses were performed to examine the stability of the results. When we assumed that the changes in standards of care might take 1 year to implement (as may occur for patients treated in lower-volume centers), total survival benefits decreased to 2.4 million years. When we examined the impact of alternative, less widely accepted estimates of the rate of vertical transmission associated with ART [32], estimated survival benefits due to pMTCT fell from 137,479 to 99,680 years.
We also relaxed the conservative estimates in sensitivity analyses. When we used the full reported efficacy of ART from the clinical trials [11, 30], per-person survival gains in ART 4 increased from 159.9 to 188.8 months, and total survival gains increased by 500,000 years. When we assumed that linkage to care was not 57% but as high as 76% [15], survival gains increased by 710,000 years. As a surrogate for earlier presentation to care, a healthier AIDS cohort-with a mean CD4 cell count of 175 cells/mm3-was also examined, since, after 1992, 70% of new AIDS diagnoses were made according to a CD4 cell count criterion of <200 cells/mm3 alone [33]. Cumulative survival benefits in this scenario increased by 740,000 years. Simultaneously relaxing all of these conservative assumptions resulted in a total survival benefit of >5.2 million years.
DISCUSSION
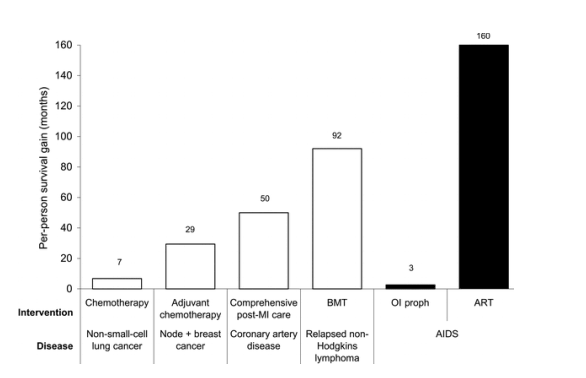
We utilized national surveillance data, efficacy data, and a state-transition probability model to estimate the survival benefits of AIDS therapy in the United States. This type of analysis does not lend itself readily to traditional forms of scientific investigation, since there is no counterfactual to the history of AIDS treatment in reality. Projected per-person survival after an AIDS diagnosis increased from 19 months (1.6 years) in the absence of treatment to 179 months (14.9 years) by 2003, a gain of 160 months (13.3 years). This survival benefit greatly exceeds that achieved for patients with many other chronic diseases in the United States [34-42]. Although this type of analysis has not been conducted for many diseases, we have synthesized data from the literature to estimate life-expectancy gains for patients with other severe and chronic diseases (figure 3). For example, chemotherapy for non-small-cell lung cancer results in an average survival benefit of 7 months, and bone marrow transplantation for relapsed non-Hodgkins lymphoma is associated with a survival benefit of 92 months [34, 41, 42]. Early in the course of the pandemic, OI prophylaxis had a profound impact on changing the face of AIDS and on shaping the perception that HIV disease was treatable [43]. However, the current magnitude of the life-expectancy gain from AIDS treatment is largely attributable to combination ART; this has transformed AIDS from a fatal disease to a highly treatable chronic condition, with average survival from AIDS diagnosis that is now >14 years.
Figure 3. Per-person survival gains for patients with various interventions for chronic diseases in the United States [34-42]. Opportunistic infection prophylaxis (OI proph) confers a 3-month benefit (if no benefit to antiretroviral therapy [ART] in those eras is assumed). ART produces 160 months of per-person survival gain by the year 2003. BMT, bone marrow transplant; MI, myocardial infarction.
As many as 312,000 of the estimated 1,185,000 people infected with HIV in the United States are thought to be unaware of their serostatus [44]. Of those who are aware of their infection, only 57% are estimated to be in care [15]. Using a cohort of patients with higher CD4 cell counts as a surrogate for earlier diagnosis and linkage to care, we found that an additional 740,000 years of life might have been saved, had all patients with AIDS in the United States received appropriate treatment on diagnosis. Thus, our findings not only demonstrate the striking survival gains achieved via advances in AIDS treatment but also emphasize the importance of expanded HIV testing and linkage to care, so that greater numbers of infected persons can access lifesaving therapy.
Previous work using the same HIV disease model estimated that, in 1996, ART led to per-person survival benefits of 18.5 months [16]. Updated results from the current analysis suggest that mean survival increases from the same era (ART 1) were 93.7 months. The additional 75.2 months reflects the value of new HIV therapies that have been developed and are available for patients receiving failing regimens. Our results are also consistent with those demonstrating the effectiveness of current ART in large cohort studies, in which the median AIDS-associated survival was found to be extended by 14.8 years [45].
This analysis has several limitations. Patients entering ART 1 often were mono-drug- or dual-drug-therapy experienced. We addressed this problem by modeling early treatment efficacy rates on the basis of patients who had received zidovudine monotherapy [5]. To match estimates in clinical cohorts rather than clinical trials, we decreased the reported efficacy rates from all trials by an additional 15% [11, 30]. Estimating the year of entry into care compared with the year of AIDS diagnosis was a challenge; we conservatively limited the eligible patients entering care each year, reduced their assumed state of health (mean CD4 cell count, 87 cells/mm3), and assumed that those who did not access care in the year of diagnosis were never eligible for care.
The analysis did not account for later ART-related toxicities that may result in, for example, cardiac disease or diabetes [46]. This exclusion is unlikely to have had a major impact on the analysis. Although the relative risk of cardiac disease may be increased as a result of ART, this increase is greatly outweighed by the absolute reduction in the risk of AIDS-related complications from ART [47]. Previous work estimating HIV treatment-induced changes in lipid levels suggests that hyperlipidemia reduces overall life expectancy by about 1 month [48].
Early reports of OI prophylaxis and ART efficacy in less-developed countries have suggested that the per-person survival gains in these settings may be comparable to those in the United States, even in the absence of customized, highly monitored care [49]. With 38 million HIV-infected people worldwide, the increased availability of ART through the WHO-sponsored "3 by 5" initiative, as well as through the President's Emergency Plan for AIDS Relief (PEPfAR) and other sources, has the potential to save hundreds of millions of years of life in the global setting and speaks to the imperative to deliver treatment to individuals in these countries quickly and efficiently [50, 51].
HIV disease has claimed >20 million lives worldwide and more than half a million lives in the United States alone [13, 51]. Our analysis demonstrates the striking scientific and clinical benefits achieved in the fight against this disease. Ten years after the introduction of potent combination ART, at least 3 million years of life have been saved in the United States.
METHODS
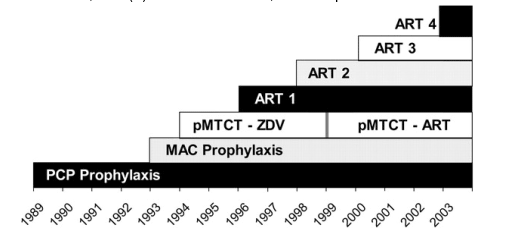
Analytic overview. We defined 6 distinct eras of HIV/AIDS treatment from 1989 to 2003, reflecting advances in opportunistic infection (OI) prophylaxis and increasingly effective ART over time (figure 1) [2-12]. Using CDC surveillance and other published data, we estimated the number of patients with AIDS who received their diagnoses and entered care each year in the United States [13-15]. We used a previously developed computer simulation model of HIV disease to assess per-person survival gains for each treatment era, compared with survival in the absence of treatment [16-18]. Cumulative survival estimates for all patients who entered care were then calculated.
Figure 1. Timeline of major HIV interventions and when they became the standard of care in the United States. Six treatment eras were defined to correspond to the availability of improved therapies and changing standards of clinical care. The first 2 eras denote advances in opportunistic infection prevention, with prophylaxis for Pneumocystis jiroveci pneumonia (PCP) starting in 1989 and prophylaxis for Mycobacterium avium complex (MAC) disease starting in 1993 [2-4]. Treatment with combination antiretroviral therapy (ART) was divided into 4 eras. ART 1 (1996-1997) marks the introduction of potent combination ART with the widespread use of protease inhibitor (PI)-based therapy [5]. ART 2 (1998-1999) includes the sequential use of nonnucleoside reverse transcriptase inhibitor-based regimens followed by PI-based unique regimens [6, 7]. From 2000 to 2002 (ART 3), 3 effective regimen options were available, with increased options for salvage through the use of resistance testing and ritonavir-boosted PIs [8, 10]. ART 4 (2003) included improved drug efficacy as reflected by increased tolerability, decreased regimen complexity, and the introduction of enfuvirtide [9-12]. We also considered 2 eras for the prevention of mother-to-child transmission (pMTCT): (1) zidovudine (ZDV) monotherapy alone, 1994-1999, and (2) combination ART, 2000 to present.
We also included 2 eras of maternal treatment for the prevention of mother-to-child transmission (pMTCT) of HIV (figure 1). Using CDC data on the number of HIV-infected women in the United States, as well as published birth, transmission, and survival rates for HIV-infected pediatric patients, we derived the number of infant infections averted and translated those into years of life saved [13, 19, 20].
This analysis was conservative; when assumptions were necessary, they were designed to underestimate the total survival benefit. For example, we limited the size of the eligible patient population to those with AIDS; we excluded the early benefits of antiretroviral mono- and dual-drug therapy when survival benefits were more limited and HIV RNA data were not available; we utilized lower estimates for rates of linkage to care; we assumed that those who did not access care in the first year of their AIDS diagnosis were never eligible; and we omitted any benefits of reduced HIV transmission from care. Sensitivity analyses were performed to examine the stability of the results in the face of alternative assumptions regarding delays in the adoption of clinical guidelines, mean CD4 cell count at presentation, the number of patients entering care, and ART efficacies.
Estimation of the sample population. Eligibility for therapy in any treatment era was limited to patients with CDC-defined AIDS; patients with non-AIDS HIV infection were excluded. Recognizing that not all patients with AIDS diagnoses receive timely care, we used national samples to estimate the proportion of patients entering care each year. National data suggest that 88% of eligible patients in the pre-ART era were receiving OI prophylaxis and that 57% of those in all of the ART eras were receiving appropriate comprehensive care [14, 15]. We characterized the newly diagnosed cohort as reflecting patients with advanced AIDS, with a mean CD4 cell count of 87 cells/mm3 (SD, 70 cells/mm3) and a mean HIV RNA level of 5.0 log copies/mL [5].
HIV disease model. The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model was used to estimate per-person survival benefits. CEPAC is a widely published computer-based state-transition simulation model of HIV disease that incorporates CD4 cell count; HIV RNA level; ART efficacy; OI incidence, treatment, and prophylaxis; and other important clinical information [16-18, 21]. "State transition" means that the model characterizes the progression of disease in an individual patient as a sequence of transitions from one "health state" to another.
Health states are defined along dimensions that are both descriptive of a patient's current well-being and predictive of future clinical events. These dimensions include CD4 cell count (>500, 301-500, 201-300, 101-200, 51-100, and 50 cells/mm3) and HIV RNA level (>30,000, 10,001-30,000, 3001-10,000, 501-3000, and <500 copies/mL) [22]. In the model, the level of HIV RNA determines the rate of CD4 cell count decline, and the absolute CD4 cell count governs the monthly risk of OIs and death [16-18, 22].
In the model, HIV-infected patients are at risk for OIs (Pneumocystis jiroveci pneumonia [PCP], toxoplasmosis, Mycobacterium avium complex [MAC] disease, disseminated fungal infection, cytomegalovirus infection, and bacterial and other infections) on the basis of their CD4 cell count [16, 23]. Patients receive the recommended standards of care in the year of their diagnosis, including regular CD4 cell count and HIV RNA laboratory tests and prophylaxis for PCP and MAC disease [24].
The model is able to accommodate a range of assumptions regarding the number of sequential lines of available ART, as well as their sequencing and efficacy. In the ART eras, patients whose CD4 cell count is <200 cells/mm3 and are in care are eligible for ART. ART functions to suppress the HIV RNA level, producing a concomitant increase in CD4 cell count [5, 7, 8, 16]. The proportion of patients achieving virologic suppression while receiving each regimen is based on rates reported in the clinical trials and adjusted for lower suppression rates observed in nonclinical trial populations [5-12]. ART failure is defined as either virologic (an observed increase in HIV RNA level over 2 consecutive months) or clinical (the development of an OI). On failure of therapy, a subsequent regimen is initiated until all regimens available in that era are exhausted; the last line of therapy is continued after failure for its independent effect on averting OIs [25]. Rates of virologic suppression for ART are era specific, as described below.
Treatment with combination ART was divided into 4 eras. ART 1 (1996-1997) marks the introduction of potent combination ART with the widespread use of PI-based therapy [5]. ART 2 (1998-1999) includes the sequential use of nonnucleoside reverse-transcriptase inhibitor (NNRTI)-based regimens followed by PI-based unique regimens [6, 7]. From 2000 to 2002 (ART 3), 3 effective regimen options were available, with increased options for salvage through the use of resistance testing and ritonavir-boosted PIs [8, 10]. ART 4 (2003) included improved drug efficacy, as reflected by increased tolerability, decreased regimen complexity, and the introduction of enfuvirtide [9-12]. The literature-reported regimen suppression rates used for each ART era are provided in table 1.
Patients who initiate treatment in one era become eligible for additional therapies later, if they survive to subsequent eras. Reflecting diminishing ART efficacy with increasing ART experience, these later regimens may differ in their suppression rates from those that would be available to patients who initiate therapy in the subsequent era.
Hypothetical patients with AIDS enter the model one at a time and are followed until death. A cohort of 1 million patients was simulated, to obtain stable results; summary statistics for the analysis included average numbers of OIs and per-person life expectancy. For each year of AIDS diagnosis from 1989 to 2003, one cohort was simulated with no treatment and the second was simulated with all treatment interventions available during that era; the 2 cohorts' average life expectancies were then compared, to obtain the net treatment benefit for the cohort that received diagnoses in that year. Survival curves generated from the model were validated against CDC-reported survival curves [13].
Model input data. Detailed data for model input are provided in table 1 [2-12, 19, 20, 22, 23, 26-29]. Briefly, OI prophylaxis efficacy is based on published data and is defined as the percentage reduction in the monthly risk of OIs: 98.2% for PCP and 77.2% for MAC disease [2-4]. Rates of virologic suppression for ART are era specific, both for the initial treatment regimen and for the subsequent regimens that are available after drug resistance or poor adherence causes the initial regimen to fail. For example, data for those patients who receive diagnoses in ART 1 (or before then, if they are still alive at the beginning of ART 1) are derived from a trial of a triple-combination regimen that reported HIV RNA suppression in 60% of patients at 24 weeks [5]. This efficacy is reflective of a cohort of patients, all of whom were pretreated with zidovudine alone [5]. Among those still alive by ART 2, efficacy is derived from a second-line efavirenz-based regimen in patients pretreated with nucleoside reverse-transcriptase inhibitors (NRTIs), which achieves 60% suppression at 48 weeks [6]. For those who remain alive in ART 3 and ART 4, third- and fourth-line treatment options become available, with efficacy derived from trials showing suppression rates of 34% at 12 weeks and 30% at 48 weeks, respectively [8, 9]. All reported efficacies are extrapolated to 48 weeks, as reported elsewhere [17]. Because the reported treatment efficacies of available regimens are from clinical trials and may overstate clinical cohort efficacies, we reduced the 48-week efficacy of suppression for each regimen by a factor of 15%. This relative reduction in efficacy represents reported differences in suppression efficacies between an observational Medicaid cohort and a clinical trial population receiving a comparable NNRTI-based regimen at a similar disease stage [11, 30].
pMTCT. To estimate survival gains attributable to pMTCT, the number of HIV-infected women in the United States was obtained from CDC surveillance data [13]. Birth rates; rates of ART utilization; HIV vertical transmission rates with no treatment (25.5%), with peripartum zidovudine treatment (8.3%), and with combination ART (3.0%); and life expectancies for HIV-infected infants are provided in table 1 [19, 20, 26, 27]. Children born HIV negative were assigned age- and race-adjusted life expectancies based on US life tables [31].
EDITORIAL
Millions of Life-Years Saved with Potent Antiretroviral Drugs in the United States: A Celebration, with Challenges
Sten H. Vermund
Institute of Global Health and Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee
In this issue of the Journal, Walensky et al. estimate the benefits that have been gained from multidrug antiretroviral therapies (ARTs) since 1989 [1]. Their finding of about 3 million years of life saved in the United States quantifies ART benefits at the population level, complementing the well-known data on plummeting US death rates and lower AIDS case report rates noted in the era of potent therapy [2, 3]. The authors' detailed sensitivity analyses, varying key estimated parameters in their models, indicate that less-conservative assumptions generate an estimate of >5 million years of life saved, a plausible "higher-end" estimate of benefit. The typical HIV-infected person now receiving potent combination ART lives at least 13-14 years longer than if he or she were to forego this therapy or if it were otherwise unavailable [1]. Quantifying the survival benefits of expanded diagnosis and modern care suggests that the economic and humanitarian benefits are greater than were hitherto appreciated.
Developing drugs, testing them without undue delay, accelerating their regulatory approval, and making them widely available have saved lives (table 1). That an average of about 200,000 persons in the United States have lived an additional year in each of the past 15 years suggests the gift given to those in need from the labor of many [1]. Drugs are discovered and developed by biochemists, pharmaceutical developers, animal modelers, formulation chemists, microbiologists, pharmacologists, and many others in the pharmaceutical industry, in academia, at research institutes, and in government. Drugs are tested for safety and efficacy by clinical-trials experts, research-study nurses, clinical-trials volunteers, community activists, government scientists and science managers, community workers, health-care providers, pharmacists, ethical-review staff, and allied health workers. After drug approval through the work of pharmaceutical companies and regulatory-oversight experts, implementation depends on health-care workers, blood bankers, social workers, mental-health professionals, substance-abuse treatment providers, journalists, science writers, medical editors, spiritual leaders, corporate and small business leaders, enlightened insurers, and family and friends of patients challenged to receive lifelong polypharmacy. (Of course, our public-health workers in health education and promotion, epidemiology, and community prevention efforts are credited, together with community prevention activists, for laboring to reduce the need for these drugs altogether.) Political and policy leaders influence research and care investments even as health activists push the system to be more responsive and efficient. Central to implementation are the HIV-infected persons themselves, who, by the tens of thousands, keep their appointments, take pills, eliminate or reduce high-risk behaviors, and support peers who struggle with the promising but complex world of daily, lifelong therapy. The model of Walensky et al. gives all of us, from our complementary disciplines, cause for celebration.
Zidovudine was the first approved antiretroviral agent, offering benefits that were exciting but, ultimately, only transient, because of the HIV drug resistance resulting from monotherapy [4-8]. Walensky et al. have assumed a small contribution from Pneumocystis jiroveci prophylaxis but no net benefit from zidovudine monotherapy alone, presumably on the basis of the results of the European Concorde study [8]. Their latter assumption is debatable [6, 9-11]. Inclusion of survival benefits from zidovudine monotherapy would increase the lives-saved calculus-another conservative bias, in any case. Dual therapy proved to be much superior to monotherapy, and triple therapy was a huge advance, in turn, over the use of 2 drugs [12, 13]. This research progress and its health impact, as documented by Walensky et al., can be seen as a continuum dating from the discovery of the syndrome in 1981 and of the virus in 1983 through the successive approval of each of the 4 drug classes since 1987 (table 1). This latter-20th-century advance in antiviral therapy has its centennial parallel in the golden era of microbiology and vaccine and drug development in the late 19th and early 20th centuries. Of course, Pasteur, Koch, and their peers were empiricists with little grasp of the microbiology known today [14]; 21st century grounding in molecular methods augurs well for future discoveries leading to an eventual cure for HIV infection, flushing out and killing virus that is latent in deep tissues. This may be a good time for our national political leaders to reconsider their decision to slow the growth of the budget of the US National Institutes of Health, now lagging behind the rate of inflation [15, 16].
Use of ART to block HIV transmission from mother to infant has virtually eliminated pediatric HIV infection as a major public-health problem in the United States and other economically prosperous nations [17, 18]. Easier-to-implement nevirapine and nevirapine-zidovudine regimens were developed that could be applied anywhere in the world, as with the "Call to Action" program (sponsored by the Elizabeth Glaser Pediatric AIDS Foundation and the Bill and Melinda Gates Foundation) and the Thai government initiative [19-22]. Drugs suitable for treating pediatric-age patients with HIV infection are readily available in the United States (table 2) but are less so in resource-limited nations.
In the late 1990s, activists cajoled the pharmaceutical industry into lower drug prices. Combined with lower drug prices due to competition from producers of generic drugs (including in the United States) (table 3), a major effort to provide ART to infected persons in developing countries began through multinational (e.g., the Global Fund to Fight AIDS, Tuberculosis, and Malaria [http://www.theglobalfund.org/en/]), bilateral (e.g., the President's Emergency Program for AIDS Relief [http://www.usaid.gov/our_work/global_health/aids/pepfarfact.html]), and national (e.g., the ahead-of-its-time program in Brazil) initiatives [23, 24]. These are lowering HIV mortality rates in resource-limited settings, just as they did earlier in the economically richer nations [25]. A lethal disease has been transformed into a chronic, manageable condition wherever health services delivery, financing, drug logistics (especially critical in rural areas and developing countries), health manpower, health policy, and health psychology are applied successfully.
Walensky et al. highlight the importance of detecting all persons infected and providing care to all those who know their HIV status [1]. Innovation is needed on many fronts. The state of North Carolina identifies acutely infected, hyperinfectious persons, to provide them with risk-reduction counseling even before antibodies are detectable [26]. Brief health education messages that are designed for clinicians to deliver within the care setting have assisted persons in HIV care to reduce their high-risk behaviors [27]. Practitioners in lower-prevalence regions suggest minimizing pretest counseling through interview-based risk triage, reserving their staff time for the essential posttest counseling sessions [28]. Rapid tests are used widely in developing-world settings to cut costs and avoid the loss to follow-up inherent in an ELISA screening (a result of the inability to provide a same-day result with an ELISA). Rapid tests are an innovation used far less often in the United States than they should be [29]. Antenatal care programs should offer "opt-out" testing-that is, HIV testing that is routine in pregnant women, excluding only those women actively requesting to forego the test [30, 31]. Efforts to increase voluntary counseling and testing and knowledge of HIV status include couples counseling to reduce marital strife and to maximize family-centered care and prevention [32-34]. These are but a few examples of innovations in the diagnosis, care, and prevention of HIV infection.
Drugs that save lives are likely to save society money, because it is cheaper to care for persons with drugs in an outpatient setting than to care for them in intensive care units, acute-care hospital beds, long-term care facilities, and hospices [35-37]. Restoring economic productivity and parent-based child care saves so-called indirect costs, and fewer emergency-department visits and hospital stays save direct costs to the health-care system. Further investment in outpatient care should emphasize voluntary counseling and testing programs for HIV diagnosis that are integrated into routine medical care [38]. This must include bridges to care for those infected. The humanitarian benefits are self-evident but may not drive investment as strongly as economic arguments can. Savings may accrue to one provider (e.g., reduced unreimbursed inpatient care expenses to a hospital or lower third-party payments), but costs may be incurred by another source (e.g., Ryan White Care Act funds). Hence, policy makers may see only their costs without knowledge of direct benefits or the savings in a different bailiwick. Early indications are that savings from outpatient management substantially outweigh the costs of the ART-based outpatient treatment programs, both here in the United States and abroad, but good data are scarce [39].
The millions of life-years saved in the United States should reinvigorate policy debates as to how best to identify HIV-infected persons in our country by offering and encouraging testing as a routine part of medical screening. We must reduce barriers to care, the first of which is the difficulty with which a test is obtained in many venues. We do not require extensive, expensive, time-consuming, and intimidating pretest counseling before screening for diabetes, for example, another disease that is lethal if unmanaged but that is controllable with lifelong medication. Yet many US guidelines demand substantial counseling infrastructures that may discourage primary-care providers from offering HIV tests as easily as they can offer a urine dipstick for glucose to screen for diabetes. Posttest counseling is essential for psychosocial assistance, a bridge to HIV-related health care, and needed to reduce high-risk behaviors, but pretest counseling can be made more efficient to reduce at least one barrier of time and money [28, 38].
We now face a daunting challenge to do better. From 3 to 5 million person-years of life have been saved for persons living in the United States from 1989 to 2003, but do we know enough about the barriers to prompt diagnosis and effective referral to care? Are we doing enough about those barriers that we do recognize? If we address systematically the barriers to testing, care, and prevention, then future modelers will describe the next 15-year period as having saved hundreds of millions of life-years, not just in North America but around the globe.
|
|
| |
| |
|
|
|