| |
Fuzeon (T-20) Use in TMC114 Study
|
| |
| |
Roche issued this press release after the FDA approval of TMC-114
U.S. FDA Approval of Boosted Protease Inhibitor Enables Potent New HIV Drug Combination with FUZEON
-- Regimen combining FUZEON and darunavir (TMC-114) shown to provide unprecedented response rates in treatment-experienced HIV patients --
NUTLEY, N.J., and MORRISVILLE, N.C. (June 23, 2006) - The approval today of a ritonavir-boosted protease inhibitor (PI), darunavir, by the U.S. Food and Drug Administration (FDA) will enable physicians and patients to create a potent new treatment combination with FUZEON (enfuvirtide) for people living with drug-resistant HIV. In pivotal clinical studies with darunavir/r, up to two-thirds of patients with extensive prior exposure to anti-HIV drugs achieved undetectable levels of HIV when the drug was used in combination with FUZEON.
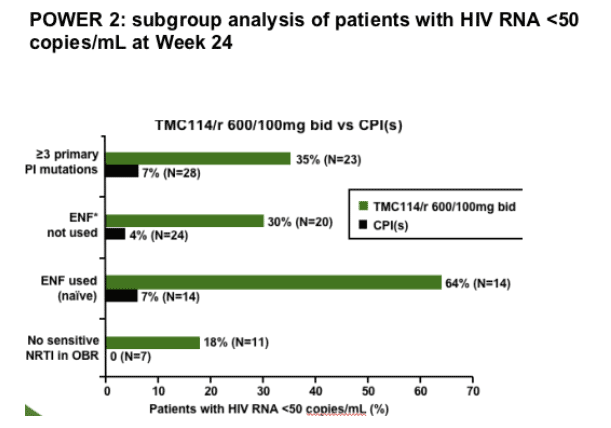
Note from Jules Levin: at the ICAAC meeting in 2005, results from the TMC114 Power 2 Study were reported & here are study results when Fuzeon was used in a regimen along with TMC114 by patients who had never used Fuzeon (64% <50 copies/ml) before & for patients who did not use Fuzeon in the power 2 Study (30%). When using TMC114 it is important to use it along with at least 1 new drug that retains full potency. This will make the regimen more potent & viral response will be more durable. If two new drugs (including TMC114) are not used for which the patient has full susceptibility there is a good chance the regimen will not succeed in providing a durable suppression of HIV RNA (viral load) & HIV drug resistance to TMC114 may develop.
Co-developed by Roche and Trimeris, Inc. (Nasdaq: TRMS) and first approved by the FDA in March 2003, FUZEON is the only fusion inhibitor for the treatment of HIV and works in a way that is different from other types of anti-HIV drugs. A product of Tibotec Pharmaceuticals Ltd., darunavir, also known as TMC-114 and the trade name Prezista■, is a member of the PI class and is reported to be active against virus that has developed resistance to other PIs.
"With the concurrent availability of FUZEON and darunavir/r, achieving undetectable HIV in a high proportion of patients with extensive prior exposure to antiretrovirals - an unprecedented result that was previously elusive to many such patients - is now not only realistic, but could quickly become a new standard of care. It is important for clinicians to recognize the potency that FUZEON, as the only fusion inhibitor, can add to regimens with duranavir/r," said Calvin J. Cohen, M.D., MSc., Research Director, Community Research Initiative of New England, Boston. "It may now be possible for many long-term HIV patients struggling against resistant HIV to achieve durable viral suppression with this combination."
The Goal of Therapy in Treatment-Experienced Patients - A Changing Standard of Care
The goal of therapy in extensively treatment-experienced patients, as defined by the Antiretroviral Treatment Guidelines from the U.S. Department of Health and Human Services (DHHS), has evolved over the last several years as newer boosted protease inhibitors, such as duranavir/r, have entered late-stage clinical trials and become available for use in combinations with FUZEON. As recently as 2003, the guidelines addressed the treatment of patients with extensive prior exposure to antiretrovirals by commenting that "viral suppression is often difficult or impossible to achieve," and emphasizing the preservation of immune function and prevention of disease progression as the primary goals of therapy in these patients. In 2005-2006,
the guidelines established maximal suppression of HIV as the goal of therapy in this patient population, and recommended the use of an entry inhibitor (such as FUZEON) with an active ritonavir-boosted protease inhibitor (such as darunavir) as a strategy for achieving that goal.
"The benefits of adding one new drug to a failing regimen could be short-lived and result in what is called 'virtual monotherapy.' It is vitally important that patients who have developed resistance to a significant number of the available antiretroviral medications start at least two fully active drugs to maximize their chances for treatment response and survival," said Nelson Vergel, an HIV treatment advocate who founded SalvageTherapies.org and has been living with the disease for more than 20 years. "This principle has been further substantiated by the excellent clinical results with the combination of FUZEON and darunavir/r."
The results achieved with FUZEON and darunavir/r add to an already substantial body of evidence supporting the use of FUZEON with an active boosted protease inhibitor as the best opportunity for treatment-experienced patients to achieve undetectable levels of HIV. Data from the FUZEON pivotal trials, TORO 1 and TORO 2, as well as the RESIST studies with tipranavir/r have consistently validated this approach.
Facts About FUZEON
Administered via one 90 mg injection twice-daily, FUZEON is the first and only fusion inhibitor for the treatment of HIV. Unlike other HIV drugs that work after HIV has entered the human immune cell, FUZEON works outside the CD4 cell, blocking HIV from entering the cell. For this reason, FUZEON is effective in treatment-experienced patients who have developed resistance to other anti-HIV drugs, though patients may still develop resistance to FUZEON. FUZEON was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in March 2003 on the basis of 24-week data, and was granted traditional (full) approval on Oct. 15, 2004 on the basis of long-term 48-week data.
Injection Site Reactions (ISRs): ISRs are the most common adverse events associated with FUZEON. ISRs occurred in 98% of patients studied and 4% discontinued FUZEON due to ISRs. Signs/symptoms may include pain and discomfort, hardened skin, redness, bumps, itching and swelling. Eleven percent of patients had local reactions that required analgesics or limited usual activities.
Pneumonia: An increased rate of bacterial pneumonia was observed in subjects treated with FUZEON in the Phase III clinical trials compared to the control arm. It is unclear if the increased incidence of pneumonia is related to FUZEON use. Patients with HIV infection should be carefully monitored for signs and symptoms of pneumonia. Risk factors for pneumonia included low initial CD4 cell count, high initial viral load, intravenous drug use, smoking and a prior history of lung disease.
Hypersensitivity Reactions: Systemic hypersensitivity reactions have been associated with FUZEON therapy and may recur on rechallenge. Hypersensitivity reactions have included individually and in combination: rash, fever, nausea and vomiting, chills, rigors, hypotension and elevated serum liver transaminases. Other adverse events that may be immune mediated and have been reported in subjects receiving FUZEON include primary immune complex reaction, respiratory distress, glomerulonephritis and Guillain-Barre syndrome.
Other Adverse Events: The events most frequently reported in patients receiving FUZEON plus an optimized background regimen were diarrhea (32%), nausea (23%) and fatigue (20%). These events were seen at a lower incidence in patients taking a FUZEON-based regimen compared to those receiving an optimized background regimen without FUZEON when taking into account the uneven number of patients in each arm and the length of time they are in that arm. As measured in number per 100 patient years, the incidence was: diarrhea (38 per 100 patient-years in subjects receiving FUZEON-based regimens vs. 73 per 100 patient-years in patients who did not receive FUZEON), nausea (27 vs. 50, respectively) and fatigue (24 vs. 38, respectively).
Please see www.tibotec.com for safety information on darunavir.
|
|
| |
| |
|
|
|