| |
Coadministration of Esomeprazole With Fosamprenavir Has No Impact on Steady-State Plasma Amprenavir Pharmacokinetics
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 42(1) May 2006 pp 61-67
Shelton, Mark J. PharmD*; Ford, Susan L. PharmD*; Borland, Julie BS*; Lou, Yu MS*; Wire, Mary B. PharmD*; Min, Sherene S. MD*; Xue, Zhengyu G. BS ; Yuen, Geoffrey PharmD*
From the *Clinical Pharmacology and Discovery Medicine; and the Experimental Pharmacogenetics Discovery and Pipeline Genetics, GlaxoSmithKline, Research Triangle Park, NC.
".....Use of prescription and over-the-counter medications that increase gastric pH (antacids, H2RAs, and PPIs) is common in HIV patients. In a survey of HIV patients, 42% receiving PI-based highly active antiretroviral therapy (HAART) regimens (n = 110) reported using prescription PPIs, 46% reported using over-the-counter PPIs or H2RAs, and 73% reported using antacids since starting HAART.16 As a result, it is important to characterize drug interactions between medications that increase gastric pH and PIs with pH-dependent solubility. This study demonstrates that simultaneous coadministration of either FPV or FPV/RTV with the PPI ESO had no impact on steady-state plasma APV pharmacokinetic parameters, including Cmax, AUC(0-ϒ), and Cϒ...... Simultaneous coadministration of ESO 20 mg qdwith either FPV 1400 mg bid or FPV 700 mg bid + RTV 100 mg bid had no effect on steady-state amprenavir pharmacokinetics. The only effect on plasma ESO exposure was a 55% increase in area under the plasma concentration-time curve during a dosing interval, ϒ[AUC(0-ϒ)], after coadministration of ESO 20 mg qd with FPV 1400 mg bid..... The 55% increase in ESO AUC after coadministration with FPV 1400 mg bid in this study is unlikely to be clinically significant based on the good safety profile of ESO at approved and higher-than-approved doses22,23 and after coadministration with clarithromycin.7 FPV 1400 mg bid and FPV 700 mg/RTV 100 mg bid, alone and in combination with ESO 20 mg qd, were generally well tolerated in this study; adverse events were generally consistent with those reported in other studies in which healthy adults received repeated doses of FPV and FPV/RTV.24-26 In conclusion, FPV 1400 mg bid and FPV 700 mg bid + RTV 100 mg bid may be coadministered simultaneously with ESO without dose adjustment...."
Abstract
Objectives: To evaluate the drug interaction between fosamprenavir (FPV) and esomeprazole (ESO) after repeated doses in healthy adults.
Methods: Subjects received ESO 20 mg once daily (qd) for 7 days followed by either ESO 20 mg qd + FPV 1400 mg twice daily (bid) or ESO 20 mg qd + FPV 700 mg bid + ritonavir (RTV) 100 mg bid for 14 days in arms 1 and 2, respectively. After a 21- to 28-day washout, subjects received either FPV 1400 mg bid for 14 days (arm 1) or FPV 700 mg bid + RTV 100 mg bid for 14 days (arm 2). Pharmacokinetic sampling was conducted on the last day of each treatment.
Results:
Simultaneous coadministration of ESO 20 mg qdwith either FPV 1400 mg bid or FPV 700 mg bid + RTV 100 mg bid had no effect on steady-state amprenavir pharmacokinetics.
The only effect on plasma ESO exposure was a 55% increase in area under the plasma concentration-time curve during a dosing interval, _[AUC(0-_)], after coadministration of ESO 20 mg qd with FPV 1400 mg bid.
Conclusions: FPV 1400 mg bid or FPV 700 mg bid + RTV 100 mg bid may be coadministered simultaneously with ESO without dose adjustment. However, the impact of staggered administration of proton pump inhibitors (PPI) on plasma amprenavir exposure is unknown at present.
Introduction
Fosamprenavir (FPV; GlaxoSmithKline, Research Triangle Park, NC), the phosphate ester prodrug of the HIV-1 protease inhibitor (PI) amprenavir (APV), is approved for treatment of HIV-1-infected adult patients. FPV has demonstrated antiviral efficacy, durability, and tolerability in antiretroviral-naive and PI-experienced subjects.1-3 FPV doses in the antiretroviral-naive studies were either FPV 1400 mg twice daily (bid) or FPV 1400 mg once daily (qd) + ritonavir (RTV) 200 mg qd FPV doses in the PI-experienced study were either FPV 700 mg bid + RTV 100 mg bid or FPV 1400 mg qd + RTV 200 mg qd RTV, also an HIV-1 PI, can be coadministered at subtherapeutic doses to increase plasma APV concentrations, primarily via inhibition of CYP3A4 metabolism.
FPV is rapidly and extensively hydrolyzed to APV during absorption, with minimal systemic FPV exposure.4 Both FPV and APV exhibit pH-dependent solubility; therefore, there is a potential for drug interactions between FPV and drugs that increase gastric pH (eg, antacids, histamine2-receptor antagonists [H2RAs], and proton pump inhibitors [PPIs]). A previous study evaluated the effects of antacids and an H2RA in combination with single doses of FPV. Simultaneous coadministration of single doses of aluminum/magnesium hydroxide (Maalox TC) 30 mL and FPV 1400 mg decreased plasma APV area under the plasma concentration-time curve (AUC) from 0 to 24 hours and maximum plasma concentration (Cmax) by 18% and 35%, respectively, but plasma APV concentration at 12 hours (C12) was increased by 14%.5 Administration of single doses of ranitidine (Zantac) 300 mg 1 hour before FPV 1400 mg decreased plasma APV area under the plasma concentration-time curve from 0 to 24 hours and Cmax by 30% and 51%, respectively, but plasma APV C12 was unchanged. The minor reduction in plasma APV pharmacokinetics with antacids was not considered to be clinically significant, but the clinical significance of the reduced plasma APV exposure after ranitidine was unclear. The effects of potent gastric acid suppression with PPIs on steady-state plasma APV pharmacokinetics are unknown.
In general, PPIs are considered to be more potent inhibitors of gastric pH compared with H2RAs based upon higher median or mean gastric pH values over a 24-hour period.6 Esomeprazole (ESO; the S-isomer of omeprazole) magnesium (Nexium; AstraZeneca, Wilmington, DE) is a potent PPI that is approved at doses of 20 and 40 mg given qd7 ESO 20 mg qd for 5 days results in mean gastric pH values of approximately 4.1 over a 24-hour period, with gastric pH remaining >4 for 53% to 56% of that interval.8,9
ESO is metabolized primarily by the cytochrome P450 (CYP) enzyme CYP2C19 and, to a lesser extent, by CYP3A4.10 Individuals may be classified as either CYP2C19 extensive metabolizers (EMs) or poor metabolizers (PMs) by phenotype, metabolism of specific phenotypic probe CYP2C19 substrates, or genotypic expression of CYP2C19 variant alleles.11 There are 7 single nucleotide polymorphisms (SNPs) that predict CYP2C19 PM status (CYP2C19*2 through CYP2C19*8), although *2 and *3 account for most of the PMs.11 Plasma ESO AUC is approximately 3-fold higher in PMs compared with EMs after the administration of single 40-mg doses of 14C-labeled ESO.12 As demonstrated with another inhibitor of CYP3A4 (ketoconazole), single-dose plasma omeprazole AUC may be increased by as much as 10-fold in PMs receiving a CYP3A4 inhibitor compared with EMs not receiving a CYP3A4 inhibitor,13 suggesting that ESO AUC in PMs might be similar or higher in this study. Because of limited, published safety data at these levels of ESO exposure, this study used the lowest approved dose of ESO 20 mg qd.
This study was designed to evaluate the effect of ESO 20 mg qd on steady-state plasma APV pharmacokinetics when coadministered with FPV 1400 mg bid and FPV 700 mg bid + RTV 100 mg bid and, conversely, the effect of FPV and FPV + RTV on steady-state plasma ESO pharmacokinetics. This study also assessed safety and tolerability of the coadministration of these drugs.
DISCUSSION
Use of prescription and over-the-counter medications that increase gastric pH (antacids, H2RAs, and PPIs) is common in HIV patients. In a survey of HIV patients, 42% receiving PI-based highly active antiretroviral therapy (HAART) regimens (n = 110) reported using prescription PPIs, 46% reported using over-the-counter PPIs or H2RAs, and 73% reported using antacids since starting HAART.16 As a result, it is important to characterize drug interactions between medications that increase gastric pH and PIs with pH-dependent solubility. This study demonstrates that simultaneous coadministration of either FPV or FPV/RTV with the PPI ESO had no impact on steady-state plasma APV pharmacokinetic parameters, including Cmax, AUC(0-_), and C_.
PPIs are considered to be more potent inhibitors of gastric pH compared with H2RAs based upon mean gastric pH over a 24-hour period; however, elevated pH may not be maintained for the entire dosing interval after repeated doses of PPIs administered qd. Although gastric pH was not measured in the current study, median gastric pH after repeated, once-daily dosing of ESO 20 and 40 mg was in the range of 2 to 3 in the morning before dosing, followed by pH values ranging from 5 to 6 (maximal) after dosing and meal administration in other studies.8,9 In contrast, median gastric pH was 6 to 7 within approximately 1 hour after administration of ranitidine 150 mg under fasting conditions.17 The FPV drug interaction study with ranitidine was designed to administer FPV at the anticipated time of maximal gastric pH (1 hour after ranitidine 300 mg under fasting conditions),5 whereas the current study used ESO and FPV in a manner that may be used in clinical practice for patients receiving both of these drugs on a long-term basis (ie, simultaneous coadministration). Thus, the gastric pH at the time of FPV administration may have been higher in the ranitidine study compared with the current study, which may account, in part, for the discrepant results between these studies in plasma APV AUC and Cmax.
Because of the potential for markedly increased ESO exposure in PMs receiving ESO in combination with FPV and FPV/RTV, the lowest approved dose of ESO (20 mg qd) was used for this study. In comparison with other PPIs, median gastric pH values with ESO 20 mg seem to be within about 0.5 pH units of those with omeprazole 20 mg and ESO 40 mg during the first several hours after PPI dosing under steady-state conditions.9 As a result, use of omeprazole or ESO 40 mg in combination with FPV or FPV/RTV is unlikely to result in a clinically significant interaction after simultaneous administration with FPV or FPV/RTV. For FPV/RTV, plasma RTV concentrations were not measured in this study because differences in RTV exposure were not expected to contribute to the potential interaction between FPV and PPIs. For the saquinavir/RTV combination, PPIs had no pharmacokinetic effect on RTV pharmacokinetics.18
Because ESO is recommended to be taken in a fasted state and FPV can be taken without regard to food intake, ESO and morning-doses FPV were coadministered simultaneously in a fasting state in this study. The impact of staggered administration of PPIs (PPI followed by FPV or FPV/RTV) or the effects of simultaneous coadministration of ESO and FPV with food on APV exposure are unknown. Although gastric pH may have been relatively low before morning dosing in this study, gastric pH was likely to be higher 12 hours after PPI dosing (values ranging from 4-6), corresponding to the time that evening doses of FPV were administered.8,9 The lack of effect of ESO on the morning predose concentrations suggests that clinically significant effects of staggered PPI administration on plasma APV trough concentrations are unlikely.
The lack of effect of a PPI on plasma APV exposure observed in this study is in contrast with those observed with atazanavir (ATV), another HIV PI with pH-dependent solubility, where marked reductions in plasma ATV exposure were noted. Repeated doses of omeprazole 40 mg qd administered in a fasted state 2 hours before ATV 300 mg/RTV 100 mg qd with a light meal were associated with a 76% reduction in plasma ATV AUC(0-_), a 72% reduction in ATV Cmax, and a 78% reduction in ATV Cmin.19 These reductions could not be overcome by coadministration with an acidic beverage (cola), nor by increasing the dose of ATV from 300 to 400 mg qd. This staggered administration strategy is consistent with the recommendations for each of these drugs because ATV should be taken with food and omeprazole should be taken in a fasted state. Based upon gastric pH profiles of omeprazole, ATV/RTV was administered at the anticipated time of maximal gastric pH.9 Recent data with an H2RA (famotidine 40 mg bid) dosed simultaneously with ATV/RTV under fed conditions resulted in a median gastric pH value of approximately 3.5 and a lower magnitude of reduction in plasma ATV exposure (14%-28%) compared with the study with omeprazole.20 Gastric pH was correlated with ATV pharmacokinetic parameters when famotidine was coadministered with ATV/RTV, but not for ATV/RTV alone. These data confirm that atazanavir is sensitive to conditions of elevated gastric pH by a variety of drugs.
Coadministration of ESO 20 mg qd with FPV 1400 mg bid increased plasma ESO AUC(0-_) by 55% and delayed t max by 1 hour; plasma ESO Cmax was unchanged. In contrast, coadministration of ESO 20 mg with FPV 700 mg/RTV 100 mg bid did not change plasma ESO AUC(0-_) and Cmax, but delayed t max by 1 hour. This observation is consistent with the results of another pharmacokinetic study in which nevirapine was coadministered with FPV with and without RTV, and plasma nevirapine exposure was increased to a greater extent by FPV 1400 mg bid than by FPV 700 mg/RTV 100 mg bid.21
The 55% increase in ESO AUC after coadministration with FPV 1400 mg bid in this study is unlikely to be clinically significant based on the good safety profile of ESO at approved and higher-than-approved doses22,23 and after coadministration with clarithromycin.7 FPV 1400 mg bid and FPV 700 mg/RTV 100 mg bid, alone and in combination with ESO 20 mg qd, were generally well tolerated in this study; adverse events were generally consistent with those reported in other studies in which healthy adults received repeated doses of FPV and FPV/RTV.24-26 In conclusion, FPV 1400 mg bid and FPV 700 mg bid + RTV 100 mg bid may be coadministered simultaneously with ESO without dose adjustment.
RESULTS
Study Participants
Fifty-six subjects (46 men, 10 women; 44 white, 9 black, 2 American Hispanic, and 1 Asian) with a mean age of 32.8 years, a mean body weight of 76.18 kg, and a mean body mass index of 24.64 kg/m2 were enrolled and included in the safety analyses. Eight subjects did not complete the study because of the following reasons: withdrawal of consent unrelated to adverse events (6 subjects), adverse events (1 subject), and nonadherence to study procedures (1 subject). Forty-eight of 56 subjects (86%) had plasma APV pharmacokinetic parameter estimates, and 53 of 56 subjects (95%) had plasma ESO pharmacokinetic parameter estimates.
CYP2C19 Genotype Status
Only the CYP2C19*2 SNP variant was observed among the study participants. Two subjects in arm 1 were homozygous for the minor allele of the *2 variant and were classified as PMs (2 of 56, 3.6%) of CYP2C19. Twenty-six subjects were heterozygous for the *2 variant and were classified as intermediate extensive CYP2C19 metabolizers (26 of 56, 46.4%). The remaining 28 subjects were homozygous for the wild-type allele for CYP2C19*2 (28 of 56, 50%) and were classified as EMs. For the purposes of categorization in this study, the 26 subjects who were heterozygous for the *2 variant and the 28 subjects who were homozygous for the wild-type allele for *2 variant were classified as EMs (n = 54).
Pharmacokinetic Analysis
Amprenavir
The median APV concentration-time curves for the 4 treatments containing FPV are presented on a linear scale in Figure 1. APV steady state was achieved by day 14 for most of these treatments, although the slope estimate of consecutive Ct values for the FPV regimen was positive at 0.039 (data not shown). The geometric mean (95% CI) values of plasma APV pharmacokinetic parameters and treatment comparisons are presented in Table 2. Confidence intervals for all APV AUC(0-_), Cmax, and C_ geometric least squares mean ratios fell between 0.75 and 1.33, the criteria for concluding no drug-drug interaction. Therefore, simultaneous coadministration of ESO 20 mg qd with either FPV 1400 mg bid or FPV 700 mg bid + RTV 100 mg bid had no effect on steady-state APV pharmacokinetics.
Esomeprazole
Achievement of steady state was not assessed for ESO-containing regimens because there were very limited numbers of samples with quantifiable ESO predose concentrations. The median ESO concentration-time curves for the treatments containing ESO are presented on a linear scale in Figure 2. The geometric mean (95% CI) values of plasma ESO pharmacokinetic parameters and treatment comparisons are presented in Table 3. Simultaneous coadministration of ESO 20 mg qd with FPV 1400 mg bid increased ESO AUC(0-_) by approximately 55%, but Cmax was unchanged. Simultaneous coadministration of ESO 20 mg qd with FPV 700 mg bid + RTV 100 mg bid resulted in no change in ESO AUC(0-_) or Cmax. For both FPV 1400 mg bid or FPV 700 mg bid + RTV 100 mg bid coadministration with ESO resulted 1 hour delay in ESO t max compared with ESO alone.
Plasma ESO AUC(0-ϒ) and Cmax were higher for the 2 PMs compared with the geometric mean values for EMs, but individual values for the PMs remained within the range of values for EMs (n = 51).
Safety Assessments
Clinical Adverse Events and Clinical Laboratory Tests
The most commonly reported clinical adverse events, regardless of intensity or relationship to study drug, included those involving the gastrointestinal, nervous, and respiratory systems (Table 4). In general, more adverse events, in particular gastrointestinal events, occurred in subjects receiving FPV-containing treatments compared with subjects receiving ESO.
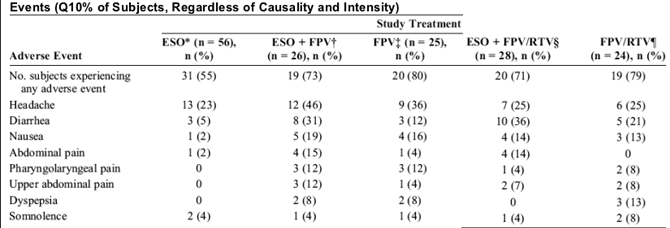

Of the 56 enrolled subjects, one subject prematurely withdrew from the study because of adverse events, including moderate heaviness in the chest, hypotension, shortness of breath, intermittent thrombocytosis, and intermittent leukocytosis during treatment with ESO + FPV/RTV. The first 3 adverse events resolved within 1 day, and the latter 2 events resolved within 5 and 21 days, respectively. There were no clinically significant changes in chemistry and hematology parameter values over time. One subject (described previously) had hypotension on day 7 of period 2 (ESO + FPV 700 mg bid + RTV 100 mg bid) that was reported as an adverse event and that spontaneously resolved; all other vital signs values were within normal limits. No clinically significant electrocardiogram values were reported.
METHODS
Study Participants
Healthy men and women (aged 18-55 years, inclusive) were eligible to participate. Subjects were informed of all aspects of the study and provided written informed consent before study participation.
Ineligibility for participation included a clinical history of alcohol or illicit drug use, history of allergy to study medications, presence of any clinically significant abnormality on medical history, physical examination, laboratory assessments, vital signs or 12-lead electrocardiogram, blood donation of >450 mL within 3 months before study entry, use of concurrent medications that could not be withheld during the study, and women who were breast-feeding or pregnant. All subjects tested negative for hepatitis B surface antigen and were seronegative for HIV, hepatitis C, and Helicobacter pylori at screening.
Study Design
This was a phase I, open-label, randomized, 2-arm, 3-period, drug-drug interaction study (GlaxoSmithKline protocol APV10031) that was conducted between July and December 2004 at one study center in the United States. All subjects were screened within 30 days of dosing to determine eligibility for enrollment into the study. Eligible subjects were randomized to arm 1 or arm 2, as shown in Table 1. Subjects were confined at the study center throughout all dosing periods. The study protocol and informed consent were reviewed and approved by the study center's Institutional Review Board.
Study Drug and Dosing
Dosing regimens are shown in Table 1; all doses of ESO were taken simultaneously with the morning doses of FPV or FPV/RTV. On serial pharmacokinetic days (day 7 of period 1 and day 14 of periods 2 and 3), subjects remained fasted for 4 hours after study drug administration (water was permitted 2 hours post dose). All doses of study drug were administered with 240 mL of water at the study center under observation by site personnel. FPV was supplied as 700-mg tablets for oral administration, RTV was supplied as oral 100-mg soft-gelatin capsules, and ESO was supplied as delayed-release capsules for oral administration. For ESO, each delayed-release capsule contained 20 mg of ESO in the form of enteric-coated pellets.
Safety Assessments
Concurrent medication use was assessed at screening, throughout the dosing periods, and at follow-up; and clinical adverse events were assessed throughout the dosing periods and at follow-up. Fasting clinical laboratory samples were collected at screening, before dosing during study treatments (days 1 and 7 of period 1, days 7 and 14 of period 2, days 1, 7, and 14 of period 3), and at the follow-up visit 21 to 28 days after discontinuation of study drug. Vital signs were collected at screening, before dosing, and at 1.5 hours after dosing on days 1 and 7 of period 1 and days 1, 7, and 14 of periods 2 and 3. Electrocardiograms were performed at screening and before dosing on day 1 of each period.
Pharmacokinetic Sampling
On serial pharmacokinetic sampling days, whole blood samples were collected at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, and 24 hours post dose on day 7 of period 1 and on day 14 of periods 2 and 3 (16- and 24-hour samples were not collected during period 3). In addition, whole blood samples were collected before morning dosing on days 4, 5, and 6 of period 1 and on days 3, 5, 7, 8, 9, 10, 11, 12, and 13 of periods 2 and 3 to establish when steady-state was achieved. Whole blood was collected in vacuum tubes containing anticoagulant (sodium citrate for APV or K3EDTA for ESO) and gently inverted to mix the blood with the anticoagulant. Blood samples were kept under refrigerated conditions for up to 1 hour before centrifugation. Plasma was separated by refrigerated (4 C) centrifugation at 2000g for 10 minutes and stored frozen at -20 C or below until analyzed for APV and ESO concentrations.
Bioanalysis of APV and ESO
Plasma samples were analyzed for APV concentrations using a validated high-performance liquid chromatography with tandem mass spectrometric detection (HPLC/MS/MS) method after solid-phase extraction at GlaxoSmithKline. Plasma samples were analyzed for ESO concentrations using a validated HPLC/MS/MS method at PPD Development (Richmond, VA).
ESO is the S-isomer of omeprazole, which is a mixture of the S- and R-isomers. Because only the S-isomer was administered in this study and the degree of inversion (conversion of S-omeprazole to R-omeprazole) in humans is negligible,14 a chiral analysis of pharmacokinetic samples was not performed. Instead, a validated assay for total omeprazole concentrations (both S-omeprazole and R-omeprazole) was used in this study. The assay calibration curves were linear from 10 to 5000 ng/mL for APV and from 1 to 1000 ng/mL for ESO. For the analyses to be acceptable, the analytical runs must have met the acceptance criteria predefined by the laboratory, which included the following: no more than one third of the quality control results must have deviated from the nominal concentration by more than 15%, and at least 50% of the results from each quality control concentration must have been within 15% of nominal.
Pharmacogenetic Assessments
A single 10-mL blood sample was collected for the analysis of CYP2C19 genotype. Nine SNPs (CYP2C19*2-CYP2C19*10) were evaluated, including the 7 SNPs known to predict CYP2C19 PM status. Genotyping was performed by a modification of the single base chain extension assay previously described.15
Pharmacokinetic Analysis
Pharmacokinetic analyses of plasma APV and ESO concentration-time data were conducted using noncompartmental methods with WinNonlin Professional software, version 4.1 (Pharsight Corporation, Mountain View, CA). APV and ESO Cmax and first time to reach Cmax(t max) were obtained from the observed values. APV and ESO area under the plasma concentration-time curves during a dosing interval, _[AUC(0-_)], were calculated using the linear up-log down trapezoidal method. The concentration at the end of the dosing interval, _(C)_), was calculated as the mean of the predose concentrations on days 11, 12, 13, and 14 of periods 2 and 3 for plasma APV concentrations.
Sample Size and Statistical Analyses
Assuming an intrasubject standard deviation of 0.29, with 80% power at _ = 0.05 and estimated treatment ratios of 1.0, it was estimated that 20 evaluable subjects per treatment arm were needed to provide a 90% confidence interval (CI) within the range of 0.75 to 1.33 for steady-state plasma APV AUC(0-_), Cmax, and C_. Assuming an intrasubject standard deviation of 0.35, 20 evaluable subjects per treatment arm were needed to provide a 90% CI about 20% around the estimated ratio of the treatment comparisons for steady-state plasma ESO AUC(0-_) and Cmax. To achieve 40 evaluable subjects, 56 subjects (28 per arm) were planned to be enrolled into the study to allow for potential subject withdrawals.
Plasma APV and ESO pharmacokinetic parameters, except t max, were log-transformed before statistical analysis. Analysis of variance, considering treatment as a fixed effect and subject as a random effect was performed using SAS (version 8.2) mixed linear models procedure (SAS Institute Inc, Cary, NC). Separate statistical models were used to compare plasma APV pharmacokinetic parameters (ESO + FPV vs. FPV and ESO + FPV/RTV vs. FPV/RTV) and to compare plasma ESO pharmacokinetic parameters (ESO + FPV vs. ESO and ESO + FPV/RTV vs. ESO). Nonparametric analysis was performed for t max using Wilcoxon signed rank test with Hodges-Lehmann estimation, and 90% CIs were provided. An indicator variable for CYP2C19 metabolizer status (PM or EM) was used as a covariate in the treatment comparison model. Achievement of steady-state plasma ESO concentrations (periods 1 and 2) and APV concentrations (periods 2 and 3) was assessed by calculating the 90% confidence interval of the slope of the linear regression of predose trough concentrations versus day collected for each treatment.
|
|
| |
| |
|
|
|