| |
Risk factors for osteonecrosis in HIV-infected patients: impact of treatment with combination antiretroviral therapy
|
| |
| |
AIDS: Volume 20(12) 1 August 2006 p 1627-1635
Mary-Krause, Muriellea; Billaud, Ericf; Poizot-Martin, Isabelleg; Simon, Anneb; Dhiver, Catherineh; Dupont, Carolinee; Salmon, Dominiquec; Roudiere, Laurentd; Costagliola, Dominiquea; the Clinical Epidemiology Group of the French Hospital Database
From the aINSERM, UMRS 720 and Universite Pierre et Marie Curie, Paris
bHospital Pitie-Salpetriere, Paris
cHospital Cochin, Paris
dHospital Necker, Paris
eHospital Ambroise Pare, Boulogne
fUniversity Hospital Centree, Nantes
gHospital Sainte-Marguerite, France.
hHospital Conception, Marseille, France.
Abstract
Background: Osteonecrosis was increasingly associated with HIV infection in the 1990s. It is unclear whether its risk increases with the duration of HIV infection, the duration of combination antiretroviral therapy (cART) or both.
Objective: To analyse factors associated with the rate of symptomatic osteonecrosis, particularly the relative impacts of the duration of HIV infection and the duration of cART, using the French Hospital Database on HIV, which comprises a large number of subjects with substantial follow-up.
Methods: Poisson regression model was used to identify factors associated with the rate of osteonecrosis among patients enrolled in 1996-2002.
Results: The study involved 56 393 subjects with a total follow-up of 229 031 person-years. Symptomatic osteonecrosis was diagnosed in 104 subjects with an incidence rate of 4.5/10 000 person-years. Multivariate analysis identified three factors associated with the rate of osteonecrosis: prior AIDS-defining illnesses [adjusted relative rate (RR), 3.1; 95% confidence interval (CI), 2.0-4.9], the CD4 cell nadir [RR, 1.6 (95% CI, 0.9-2.9) for CD4 cell count 50-199 cells/ml; RR, 1.8 (95% CI, 1.0-3.3) for CD4 cell count < 50 cells/ml; both relative to CD4 cell count ≥ 200 cells/ml] and exposure to cART. Compared with unexposed patients, the RR of osteonecrosis ranged from 2.6 (95% CI, 1.2-5.9) in patients treated with cART for < 12 months to 5.1 (95% CI, 2.1-12.6) in patients treated for ≥ 60 months.
.....It has been postulated that the association between osteonecrosis and cART may simply be a result of the immunological and virological improvement related to treatment or that it may rather be a metabolic complication of cART [7], lipodystrophy [26,39-40] or hyperlipidaemia [6], which is itself a risk factor for osteonecrosis [25]......
Conclusions: Osteonecrosis appears to be a complication of both HIV infection and cART.
Results
Among the 56 393 HIV-infected patients enrolled in this study, 28.4% were females, and the median age was 35.5 years (Table 1). The principal HIV transmission groups were homo/bisexual and heterosexual. The median known duration of HIV infection was 3.7 years; 20.5% of patients had had an AIDS-defining event, and the median nadir CD4 cell count since enrollment in the FHDH until the study entry was 242 cells/ml. At baseline, 36% of the study population were receiving at least one antiretroviral drug (median cumulative exposure 3.9 months).
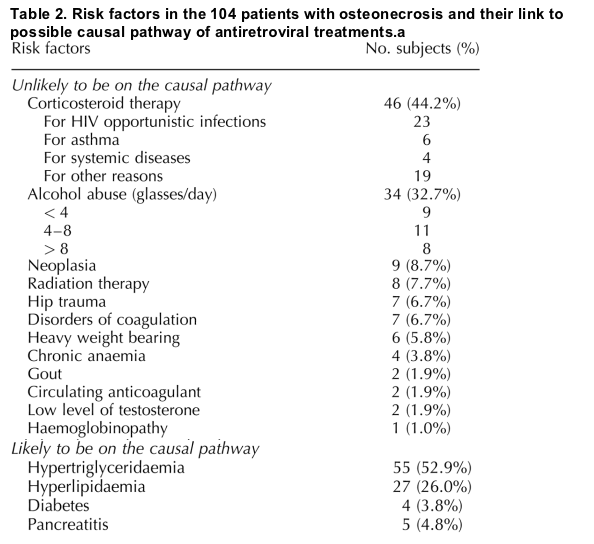
Osteonecrosis was diagnosed in 104 subjects, corresponding to an incidence rate of 4.5/10 000 person-years [95% confidence interval (CI), 3.7-5.4] and affected the hip in 83 (79.8%), the knee in 14 (13.5%) and other joints in 16 patients (15.4%). Of these patients, 63 received analgesics and 52 were treated surgically. When risk factors were considered, 12 patients with osteonecrosis (11.5%) had no risk factors, 34 (32.7%) had one category-1 risk factor, 4 (3.9%) had one category-2 risk factor, and 54 (51.9%) had at least one risk factor from both categories. Corticosteroid therapy was the most frequent risk factor, followed by excessive alcohol consumption (Table 2). There is no consensus in the literature on the role of smoking, which was notified for 58 patients (11 previous smokers and 47 current smokers). At the time of osteonecrosis diagnosis, the median CD4 cell count was 298 cells/ml (interquartile range, 179-456). Among the 99 subjects for whom plasma HIV RNA was collected in the FHDH, viral load was ≦ 500 copies/ml in 57.6% of the patients, 501-5000 copies/ml in 15.2%, 5001-30 000 copies/ml in 10.1% and > 30 000 copies/ml in 17.2%. Univariate Poisson regression analysis showed that AIDS-defining events were significantly associated with the subsequent rate of osteonecrosis [relative rate (RR), 2.4; 95% CI, 2.2-2.6). There was a non-linear relationship between the known duration of HIV infection and the risk of osteonecrosis (Fig. 1a). This could be explained by a large proportion of patients who access care only at an advanced stage of HIV disease [17]. However, the relation between the nadir CD4 cell count since enrollment in the FHDH until the first event (osteonecrosis, death or last follow-up) and the rate of osteonecrosis was monotonous (Fig. 1b). The lower the nadir CD4 cell count, the longer the duration of HIV infection. On the basis of these results, only prior AIDS-defining illnesses and the nadir CD4 cell count were included in the multivariate Poisson regression model. Univariate analysis showed that the incidence of osteonecrosis increased with the duration of cART (Fig. 1c). Osteonecrosis was also more frequent among all men, homo/bisexual men and subjects younger than 60 years.
In multivariate analysis of the variables described above, gender, the HIV transmission group and age did not significantly influence the rate of osteonecrosis (data not shown). Osteonecrosis was more likely to occur in patients with prior AIDS-defining illnesses (RR, 3; Table 3). The incidence of osteonecrosis tended to be higher among subjects with nadir CD4 cell counts of < 200 cells/ml and was significantly higher among subjects with < 50 cells/ml [RR, 1.6 (95% CI, 0.9-2.9) for 50-199 cells/ml; RR, 1.8 (95% CI, 1.0-3.3) for < 50 cells/ml]. The incidence of osteonecrosis was also higher among subjects exposed to cART. The adjusted RR ranged from 2.6 among patients treated with cART for < 12 months (compared with patients unexposed to cART), to 5.1 among patients treated for ≥ 60 months.
Discussion TOP
To our knowledge, this is the first adequately powered study to show that the incidence of osteonecrosis in HIV-infected patients increases with the duration of both HIV infection and cART treatment.
All cases of osteonecrosis were retrospectively validated, and 86.5% of diagnoses were confirmed. This analysis included both confirmed and presumptive diagnoses, but the results were similar when presumptive cases were excluded: RR ranged from 2.8 (95% CI, 1.2-6.6) among patients treated with cART for < 12 months (compared with patients unexposed to cART) to 4.9 (95% CI, 1.9-12.9) among patients treated for ≥ 60 months. It is possible that some cases were not reported. Indeed, one study showed that 4.4% of 339 asymptomatic HIV-infected individuals screened for osteonecrosis using MRI had asymptomatic osteonecrosis [18]. Nevertheless, there is no reason why the rate of undernotification should be different according to the duration of either cART or HIV infection. Consequently, only symptomatic osteonecrosis was taken into account in this study.
In our study, osteonecrosis was considered confirmed if it was diagnosed by MRI and/or scintigraphy and/or surgery. Radionuclide imaging is superior to conventional radiography in the detection of avascular necrosis but is less sensitive that MRI, which is currently the preferred imaging technique in this setting. Nevertheless, the sensitivity for detection of avascular necrosis has ranged from 72% to 87% for radionuclide bone scanning while it is 88% to 100% for MRI [19]. In the typical patient presenting with hip pain, there have been no studies to indicate that MRI should be used routinely to detect occult avascular necrosis. Because of the higher cost of MRI, bone scintigraphy is one of the most frequently performed of all radionuclide procedures and is very useful for localizing avascular necrosis in patients with negative radiographs [20]. One study in which a review of 524 studies on osteonecrosis was performed, with 213 selected and cited, showed that skeletal scintigraphy and MRI had enhanced diagnosis greatly [21]. In our study, diagnosis of osteonecrosis was made on bone scintigraphy without MRI for five patients. In one of them, a bone biopsy was performed. Bone scanning associated with standard radiography was also performed for one patient and a standard radiography alone was provided for another. If we considered these five patients as presumptive diagnoses, the results obtained based solely on MRI-confirmed diagnosis are similar to those obtained in the analysis included both MRI and scintigraphy to confirm diagnosis: RR ranged from 3.2 (95% CI, 1.3-7.8) among patients treated with cART for < 12 months (compared with patients unexposed to cART) to 4.6 (95% CI, 1.6-12.7) among patients treated for ≥ 60 months.
It is noteworthy that the incidence of osteonecrosis, stratified for the duration of cART and the year of cART outset did not increase with time in this cohort. For cART durations of 12-23 months, for example, the incidence rates per 10 000 person-years were 8.3 ± 3.2, 8.9 ± 3.2, 2.9 ± 2.0, 8.8 ± 4.4, 6.4 ± 4.5 when cART was started in 1996, 1997, 1998, 1999 and 2000, respectively. Similar fluctuations were observed for other durations of exposure to cART. Therefore, our results are unlikely to be explained by increased notification of osteonecrosis in recent years.
Furthermore, our results are unlikely to be a consequence of remaining confounding. To be confounding, a factor must be linked both to the studied disease and to the variable under study, for instance the duration of cART. Category-1 risk factors for osteonecrosis were unlikely to be related to the duration of cART, except for corticosteroid therapy [22-24], which is prescribed for a variety of disorders, particularly in the advanced stages of HIV infection. Our multivariate analysis took this potential confounding factor into account as we adjusted for AIDS-defining events and the nadir CD4 cell count. Therefore, the impact of this factor, if it exists, is probably small.
Risk factors such as hypercholesterolaemia, hypertriglyceridaemia, diabetes and pancreatitis are potentially related both to HIV infection [25,26] and to its treatment [27]. Rather than being confounding factors, they are more likely to be on the causal pathway of a potential antiretroviral treatment effect. Including time-updated levels of cholesterol and triglycerides in the model could reduce the association of duration of exposure to cART with osteonecrosis without removing it [28].
We found that prior AIDS-defining events, the nadir CD4 cell count and the duration of cART independently influenced the risk of osteonecrosis. Clearly, the duration of treatment could be linked to the duration of HIV infection. Indeed, the longer the subjects were infected, the more likely they were to have a long duration of treatment.
Osteonecrosis has been reported among untreated HIV-infected patients and also among patients receiving zidovudine monotherapy [5,29]. The duration of known HIV seropositivity is not a good indicator of the duration of HIV infection, as more than 30% of newly enrolled patients in the FHDH have CD4 cell counts < 200 cells/ml or an AIDS-defining disease at baseline [17]. This is why we used prior AIDS-defining events and the nadir CD4 cell count to adjust the Poisson regression model: these are more appropriate to represent the duration of HIV infection. Indeed, the more patients are exposed to HIV infection, the more the CD4 cell count falls, the lower the nadir CD4 cell count and the more likely an AIDS-defining event is to occur. We found an impact of both the duration of cART and the duration of HIV infection on the incidence of osteonecrosis, although an interaction between these two variables cannot be excluded.
High levels of anticardiolipin antibodies have been implicated in the occurrence of osteonecrosis in HIV-infected patients but this is controversial [22,30]. Other authors have implicated HIV infection itself, through increased production of proinflammatory cytokines such as interleukin-6 and tumour necrosis factor [5]. A published letter has suggested that bone might be a sequestered reservoir of HIV, and that HIV in bone might effect the depletion of CD4 cells in bone marrow [31]. A link between osteonecrosis and steroid treatment of AIDS-related disorders has also been suggested [30,32]. Moreover, although there is no consensus on the role of smoking as a risk factor for osteonecrosis, Newman et al. [33] have reported that smokers have a fourfold-higher risk of osteonecrosis, probably related to smoking-induced vasospasm, and it is noteworthy that HIV-infected patients tend to smoke more than the general population [34].
Some studies have suggested a link between the risk of osteonecrosis and exposure to protease inhibitors [5-7], while others have not [22,35-38]. Some of these studies were simple case reports [7,36], and others suffered from methodological issues such as small study populations, few controls unexposed to cART and frequent use of protease inhibitors, possibly simply reflecting general practice in the cART era.
It has been postulated that the association between osteonecrosis and cART may simply be a result of the immunological and virological improvement related to treatment or that it may rather be a metabolic complication of cART [7], lipodystrophy [26,39-40] or hyperlipidaemia [6], which is itself a risk factor for osteonecrosis [25].
On the one hand, it would be interesting to evaluate the potential impact of specific antiretroviral drugs on the occurrence of osteonecrosis. On the other hand, evaluating the role of a therapeutic class such as protease inhibitors may not be relevant, as the different drugs within a given class do not all have the same side-effects. For instance, stavudine is more strongly associated with mitochondrial toxicity [41-43], while zidovudine is more associated with anaemia. Some protease inhibitors have been associated with increased insulin resistance or abnormalities of lipid metabolism, while others have not [27]. However, accounting for exposure to each drug would be extremely difficult for two reasons: first, it would decrease the power of the study, and second, and more importantly, it is difficult to document precisely the duration of exposure to each drug used as part of cART.
In conclusion, this study shows that the risk of symptomatic osteonecrosis increases with the duration of both HIV infection and antiretroviral therapy. Reporting bias and confounding factors are unlikely to explain the observed results. Although osteonecrosis is a relatively uncommon condition, the risk of osteonecrosis must be borne in mind among patients with bone pain, and especially in patients with advanced HIV disease or lengthy cART exposure, even when there are no known risk factors.
Introduction
The advent of increasingly effective antiretroviral drugs has reduced the morbidity and mortality of HIV infection [1,2]. Osteonecrosis, also known as avascular necrosis and aseptic necrosis of bone, was increasingly associated with HIV infection in the 1990s [3,4]. Results of case reports and case series have suggested that the incidence of osteonecrosis may be increasing since the advent of combination antiretroviral therapy (cART) [5-7], possibly owing to the side-effects of such treatment, particularly protease inhibitors [8-10], even if abnormalities of lipid metabolism in HIV-infected patients were described well before the advent of cART [11,12]. It is not known whether the incidence of osteonecrosis increases with the duration of HIV infection, the duration of cART or both.
The French Hospital Database on HIV (FHDH, ANRS CO 04), which includes a large number of subjects with substantial follow-up, offers an opportunity to analyse factors associated with the rate of osteonecrosis in this setting, and especially the relative impacts of the duration of HIV infection and the duration of cART.
Methods
Study population
The FHDH clinical epidemiological network was created in 1992 and involves 62 French teaching hospitals belonging to 29 HIV/AIDS treatment and information centres (CISIH). It is one of the largest cohorts of HIV-infected patients in the world. The only enrollment criteria are confirmed HIV infection and written informed consent. Data are collected prospectively by trained research assistants, using DMI2 software (property of the French Ministry of Health). The standardized data collection form includes questions on the transmission group, standard biological markers such as the CD4 cell count and plasma HIV RNA level, pathologies occurring during follow-up (coded with the International Classification of Diseases), antiretroviral treatments and prophylaxis, clinical trials in which the patient is enrolled, and death and cause of death (as recorded in the medical records). A follow-up form is completed at least every 6 months and/or at each visit or hospital admission during which a new pathology is diagnosed, a new treatment is prescribed or a change in biological markers is noted.
A combination of three or more antiretroviral drugs is considered to be cART. During the study period, cART potentially consisted of a combination of two nucleoside analogue reverse transcriptase inhibitors to which was added at least one protease inhibitor (boosted or not), a non-nucleoside analogue reverse transcriptase inhibitor or a third nucleoside analogue reverse transcriptase inhibitor. Protease inhibitors became widely available in France in late March 1996, and non-nucleoside analogue reverse transcriptase inhibitors in late 1998. Entry to this study was thus defined as the date of the first CD4 cell count after 1 January 1996. Among the 94 350 patients aged at least 15 years who were enrolled in the FHDH cohort between 1992 and 2002, 66 429 had at least one follow-up visit and one CD4 cell count between 1996 and 2002. Of these subjects, 56 785 (85.5%) had at least two follow-up visits after entry to the study, or died before the second visit or hospital admission. Patients were not eligible for this study if they had a history of osteonecrosis or ongoing osteonecrosis at FHDH enrollment, or if they were receiving antiretroviral treatment in a double-blind trial. A total of 56 411 (84.9%) individuals met these criteria, of whom 122 developed osteonecrosis during follow-up.
Definition of osteonecrosis
Osteonecrosis was coded 730.1 (chronic osteomyelitis) or 733.4 (aseptic necrosis of bone) prior to 1997 [13], and M87 (osteonecrosis) thereafter [14].
For each case of osteonecrosis, a questionnaire was sent to the relevant participating centre to validate the diagnosis and to collect data on any preexisting risk factors that were not recorded in the FHDH. Osteonecrosis diagnosed by magnetic resonance imaging (MRI) and/or scintigraphy and/or surgery was considered confirmed. Diagnoses based only on computed tomography, standard radiography or clinical findings were considered presumptive. All presumptive and confirmed cases were included in this analysis. Osteonecrosis was diagnosed in 122 patients. Following validation, 13 subjects (10.7%) were excluded from the analysis because they did not have osteonecrosis, and five (4.1%) were excluded because a past history of osteonecrosis was discovered. Therefore, the study population consisted of 56 393 subjects with a total follow-up of 229 031 person-years; in this group there were 90 confirmed (86.5%) and 14 presumptive diagnoses of osteonecrosis.
Candidate risk factors were divided into those probably related (category 1) and those probably unrelated (category 2) to antiretroviral treatment. Category 1 included alcohol abuse, chronic anaemia, circulating anticoagulant, corticosteroid therapy, clotting disorders, gout, obesity, haemoglobinopathies, cancer, radiation therapy, cancer chemotherapy, systemic lupus erythematosus, trauma with fracture or microfracture, and vasculitis. The second category included hypercholesterolaemia, hypertriglyceridaemia, diabetes mellitus and pancreatitis.
Statistical analysis
All analyses were based on incidence rates calculated from study entry to study end, the latter being defined by the date of the first event (osteonecrosis, death or last follow-up). Only events that occurred between study entry and the end of 2002 were analysed. To take reporting delays into account, a censoring method recommended for cohort studies was used [15]. Surviving patients for whom the last follow-up data were obtained in the 6-month period before 31 December 2002 (last database update) were censored on 31 December 2002.
A Poisson regression model was used to identify factors associated with the rate of osteonecrosis. To study the impact of the duration of HIV infection, the date of infection is clearly required. As this date is generally unknown, three surrogates determined at time of the first event were tested: the known duration of HIV infection, the CD4 cell nadir since enrolment in the FHDH and prior AIDS-defining events. AIDS was defined by using the clinical criteria of the 1993 European revised case definition [16].
To analyse the relation between the incidence of osteonecrosis and the duration of cART, each patient's follow-up was divided into 1-month periods. Cumulative exposure to cART was calculated at the start of each period, and the result was used to assign the patient-month (and any endpoint events that occurred during that month) to the appropriate exposure category. The exposure categories were: no exposure to cART (but possible exposure to single- or dual-agent therapy), and exposure for < 12, 12-23, 24-35, 36-47, 48-59 and ≥ 60 months. If a person discontinued therapy, his or her exposure category remained what it had been at the time of discontinuation. Osteonecrosis occurring after treatment discontinuation was entered in the last category of exposure. Person-years after cART cessation were not taken into account when evaluating the duration of exposure to cART or the duration of no exposure to cART.
Multivariate analysis included only variables with P values < 0.2 in univariate analysis. These comprised gender, age at osteonecrosis diagnosis or at the end of follow-up, and the HIV transmission group. All analyses used SAS statistical software version 8.2 (SAS Institute, Cary, North Carolina, USA).
|
|
| |
| |
|
|
|