| |
Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients
|
| |
| |
[Research Letters]
AIDS: Volume 20(12) 1 August 2006 p 1675-1677
Coll, Blaia,b; Aragones, Gerarda; Parra, Sandrab; Alonso-Villaverde, Carlosb; Masana, Lluisb
aCentre de Recerca Biomedica, Reus, Spain
bServei de Medicina Interna, Hospital Universitari Sant Joan, Reus, Spain.
Abstract
We tested the security and efficacy of ezetimibe in the treatment of HIV-associated dyslipemia. Twenty HIV-infected patients were randomly assigned to receive ezetimibe 10 mg/day or fluvastatin 80 mg/day. Patients receiving ezetimibe experienced a statistically significant (P = 0.003) 20% reduction in the concentration of LDL-cholesterol, similar to that observed with fluvastatin (24%, P between groups 0.70). We concluded that ezetimibe monotherapy effectively decreases LDL-cholesterol in HIV-infected patients.
The control of cardiovascular risk factors in HIV-infected patients is relevant because the incidence of myocardial infarction [1] and other atherosclerosis-related events [2] are increasing. Lipid abnormalities are commonly present in this clinical setting [3], but the majority of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, with the exception of pravastatin and fluvastatin, are metabolized by the cytochrome P450 3A4, which in turn is also the metabolic pathway of many of the antiretroviral agents, especially the protease inhibitors (PI) [4]. Recent evidence supports the use of ezetimibe in combination with statins to add a powerful lipid-lowering effect [5]. However, the metabolism of ezetimibe, which is P450 independent, and the low incidence of drug interactions and side-effects, make this drug suitable to be tested in monotherapy in HIV-infected patients.
Patients were enrolled during the 3 months of the inclusion period if they fulfilled the eligibility criteria: more than 6 months on stable HAART, more than 18 years of age, and a fasting LDL-cholesterol concentration of 3.30 mmol/l or greater. Classic cardiovascular risk factors were recorded, and fasting total cholesterol, HDL-cholesterol, triglycerides, and glucose were determined. LDL-cholesterol values were obtained using the Friedewald formula. CD4 and CD8 lymphocyte counts and HIV-1 viral load were determined using standard techniques.
Endothelial function was analysed using peripheral arterial tonometry [6]. Briefly, this system (Itamar Medical Ltd., Caesarea, Israel) utilizes a finger probe to assess digital volume changes accompanying pulse waves. The peripheral arterial tonometry data were analysed by a computer in an operator-independent manner. A ratio of less than 1.6 was considered to be a marker for endothelial dysfunction [6].
Patients were then randomly assigned (according to the HAART regime: boosted PI or non-nucleoside analogues) to receive ezetimibe 10 mg/day or fluvastatin extended release 80 mg/day in a 1: 1 ratio. Patients were evaluated 2-3 weeks after the initiation of lipid-lowering agents to assess tolerability and adherence, and at 6 weeks, the baseline protocol was re-applied.
Results are expressed as mean (SEM) or in percentages. Univariate analyses, using non-parametric tests, were used to compare differences between groups (Mann-Whitney) and between pre and posttreatment (Wilcoxon and McNemar tests). The independent Ethics Committee of the Hospital Universitari Sant Joan approved the study.
There were no significant differences in lipid values, age, and baseline endothelial function between patients taking non-nucleoside analogues (N = 10) and those with boosted PI (N = 10). Patients randomly assigned to receive ezetimibe exhibited higher systolic [137 (5.6) versus 117 (2.7) mmHg, P = 0.01] and diastolic blood pressure [90 (3.3) versus 76 (4.5) mmHg, P = 0.02] than those on fluvastatin. There were no significant differences either in the other cardiovascular risk factors or in the HIV-related variables between groups, although most of the patients assigned to be given ezetimibe were receiving lopinavir/ritonavir. None of the participants experienced related side-effects and none of them interrupted the lipid-lowering therapies.
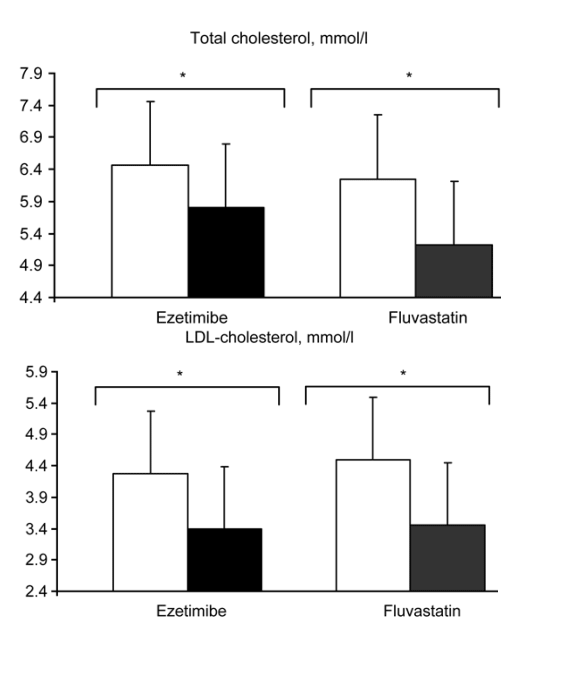
In both groups, total cholesterol and LDL-cholesterol were significantly decreased after therapy (Fig. 1). Those patients receiving ezetimibe 10 mg presented a statistically significant (P = 0.03) 10% reduction in total cholesterol and a 20% reduction in LDL-cholesterol concentrations (P = 0.02, Fig. 1). The results in the fluvastatin group were similar, showing a 17% reduction in total cholesterol (P = 0.06) and a 24% decrease in the concentrations of LDL-cholesterol (P = 0.02). We did not find significant differences in either triglycerides or HDL-cholesterol (Fig. 1). There were no significant differences in the reduction of lipid concentrations between groups, showing a similar effect of both lipid-lowering agents (Wilcoxon test, P = 0.2 for total cholesterol and P = 0.4 for LDL-cholesterol).
Furthermore, those patients receiving ezetimibe did not exhibit significant changes in endothelial function after 6 weeks of therapy (Fig. 1). However, those in the fluvastatin group experienced a non-significant (P = 0.5) increase in the rate of endothelial function (11% better result in the second evaluation, Fig. 1).
The treatment of HIV-associated dyslipidemia should avoid drug-drug interactions and potentially detrimental concentrations of statins [7-10]; therefore, the use of lipid-lowering agents without significant interactions with HAART is highly desirable.
Pravastatin is not preferentially metabolized by cytochromes [10], and fluvastatin is metabolized through the CYP2C9 enzyme. Both reduce the concentrations of total cholesterol without significant toxicities [10,11], but other lipid-lowering drugs should be evaluated in this particular group of patients in order to control potential toxicities. Ezetimibe is the first available selective cholesterol absorption inhibitor, blocking cholesterol absorption at the intestinal brush border to reduce LDL-cholesterol [12]. The dual inhibition of cholesterol synthesis and absorption, through the co-administration of a statin and ezetimibe, has been shown to provide significantly greater reductions in LDL-cholesterol than statin monotherapy alone [5]. However, if confirmed, we also support the use of ezetimibe in monotherapy in HIV-infected patients, because it yields similar results to those obtained with fluvastatin, and none of the participants experienced any ezetimibe-related side-effects.
The small sample size and the different distribution of the boosted PI between groups are severe limitations to be considered. Even then, the results favour the use of ezetimibe to control lipid-related cardiovascular risk factors.
An important issue addressed in our study was the influence of these agents on endothelial function; HIV-infected patients exhibit higher rates of endothelial dysfunction than the non-infected population [13], and those on PI have even worse values [14]. Agents that ameliorate endothelial function contribute to a reduction in cardiovascular risk, because endothelial dysfunction is the initial disturbance in the development of atherosclerosis [15]. Those patients on ezetimibe, although reducing significantly the values of LDL-cholesterol, did not experience any change in endothelial function. Conversely, patients receiving fluvastatin ameliorated, although not significantly, their endothelial function.
In summary, ezetimibe monotherapy effectively decreases LDL-cholesterol in HIV-infected patients. Because of the small sample size, these results should be further addressed in larger trials, but the use of ezetimibe in these patients can be advised.
Sponsorship: This study was financially supported by the Fondo de Investigacion Sanitaria (FIS PI041752) and RC/MN (C03/08). B.C. is the recipient of a career development award from the Instituto de Salud Carlos III.
Fig. 1. Results of lipid parameters and endothelial function in HIV-infected patients before (white bars) and after (filled bars) 6 weeks of ezetimibe ( N = 10) or fluvastatin ( N = 10). *P < 0.05 comparing pre and post-treatment assessment (Wilcoxon test).
|
|
| |
| |
|
|
|