| |
Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors
|
| |
| |
American heart Journal
Volume 147, Issue 4, Page 713 (April 2004)
James H. Stein a, Michelle A. Merwood a, Jennifer L. Bellehumeur a, Susan E. Aeschlimann a, Claudia E. Korcarz a, Gail L. Underbakke a, Maureen E. Mays a and James M. Sosman a
Received 8 August 2003; accepted 16 October 2003.
"......short-term therapy with pravastatin safely reduced total cholesterol, LDL cholesterol, and non-HDL cholesterol levels. Lipoprotein changes included reductions in the concentrations of LDL particles and small VLDL particles. These changes were associated with a trend toward improved FMD of the brachial artery......more aggressive lipid-lowering therapy will be needed to improve endothelial function....Combinations of lipid-lowering therapies may be needed to normalize the lipoprotein abnormalities and endothelial dysfunction observed in patients taking HIV PIs.."
Abstract
Background
Although recommended as initial therapy for patients with dyslipidemia who are taking human immunodeficiency virus protease inhibitors (HIV PIs), the effects of pravastatin on lipoproteins and arterial reactivity have not been elucidated. The purpose of this study was to determine the effects of pravastatin on lipoprotein subfractions and endothelial function in patients with dyslipidemia who are receiving HIV PIs. (Use of HIV PIs: Amprenavir
Indinavir, Nelfinavir, Ritonavir (5 Lopinavir/ritonavir, saquinavir/ritonavir, 1 indinavir/ritonavir), Saquinavir)
Methods
This was a placebo-controlled, double-blind, crossover study comparing pravastatin (40 mg) to placebo in 20 patients who were taking HIV PIs. Lipoprotein subfractions were measured with nuclear magnetic resonance spectroscopic analysis. Flow-mediated vasodilation (FMD) of the brachial artery was evaluated with high-resolution ultrasound scanning.
Results
At baseline, subjects had an increased concentration of low-density lipoprotein (LDL) particles (1756 ± 180 nmol/L), which tended to be small (19.9 ± 0.2 nm), a low concentration of large high-density lipoproteins (HDL; 0.94 ± 0.07 mmol/L), and an increased concentration of large very low-density lipoproteins (VLDL; 1.90 ± 0.58 mmol/L). FMD was impaired (4.5% ± 1.1%). Compared with placebo, pravastatin resulted in a 20.8% reduction in LDL particles (P = .030), a 26.7% reduction in small LDL (P = .100), and a 44.9% reduction in small VLDL (P = .023). Total and non-HDL cholesterol levels decreased by 18.3% (P <.001) and 21.7% (P <.001), respectively. FMD tended to increase in patients receiving pravastatin (0.7% ± 0.6%); however, the difference between treatment phases was not statistically significant (P = .080).
Effects of pravastatin
Compared with placebo, pravastatin reduced total cholesterol levels by 18.3% (P <.001), LDL cholesterol levels by 20.4% (P = .022), and non-HDL cholesterol levels by 21.7% (P <.001). Lipid changes observed with pravastatin were characterized by a 20.8% reduction in the LDL particle concentration compared with placebo (P = .030). With pravastatin, there was a 26.7% reduction in the concentration of small LDL particles (P = .100) and a 44.9% reduction in the small VLDL concentration (P = .023); however, mean LDL, VLDL, and HDL particle diameters did not change significantly. Significant changes in daily intake of calories, calories from fat and saturated fat, cholesterol, fiber, soluble fiber, or alcohol were not observed (Table III).
The brachial artery diameter did not change significantly in treatment phases. Heart rates (P = .141), systolic blood pressure (P = .917), baseline forearm blood flow (P = .146), and blood flow during reactive hyperemia (P = .766) also did not differ in treatment phases (data not shown). FMD increased with pravastatin (0.7% ± 0.6%, P = .080). After adjustment for small differences in baseline brachial artery diameters in treatment phases, the improvement in FMD with pravastatin did not achieve statistical significance (P = .087). The change in FMD with pravastatin was similar in subjects with and subjects without hypertension. In multivariable analysis, predictors of improvement of FMD with pravastatin included glucose (P = .004), IDL (P = .001), and HDL cholesterol (P = .042) levels. The final model explained a modest amount of the change in FMD with pravastatin therapy (FMD CHANGE = -0.18 + 1.63 X glucose - 4.41 X IDL + 9.7 X HDL cholesterol; r2adjusted = 66.4%, P <.001).
Conclusions
This is the first double-blind, placebo-controlled study of the effects of statin therapy on lipids, lipoprotein subfractions, and endothelial function in patients taking HIV PIs. Pravastatin reduced concentrations of atherogenic lipoproteins, particularly those most associated with future coronary events.
Discussion
This is the first placebo-controlled, double-blind evaluation of the effects of pravastatin on lipids in patients receiving highly active antiretroviral therapy, and the first to evaluate its effects on lipoprotein subfractions and endothelial function. In this study, short-term therapy with pravastatin safely reduced total cholesterol, LDL cholesterol, and non-HDL cholesterol levels. Lipoprotein changes included reductions in the concentrations of LDL particles and small VLDL particles. These changes were associated with a trend toward improved FMD of the brachial artery.
The lipoprotein changes observed with pravastatin were similar to those reported in a prospective trial of patients not infected with HIV who had coronary artery disease, which demonstrated that therapy with pravastatin reduced the incidence of adverse coronary events and coronary angiographic progression (Pravastatin Limitation of Atherosclerosis in the Coronary Arteries; PLAC-I).21, 22 In the PLAC-I trial, the LDL particle concentration was predictive of coronary angiographic progression in subjects who received placebo, but not subjects treated with pravastatin, indicating that the salutary effects of lipid-lowering therapy on angiographic progression are mediated, in part, by decreasing the concentration of circulating LDL particles.22 In the current study, the LDL particle concentration decreased with pravastatin therapy. Most of the LDL reduction was attributable to a decrease in the concentration of small LDL particles, which are associated with increased cardiovascular risk and progression of angiographic coronary artery disease.23, 24, 25 Concentrations of small VLDL also are associated with increased coronary atherosclerosis.18 Overall, the salutary changes in lipids observed during pravastatin therapy reflected reductions in atherogenic lipoproteins and would be expected to reduce coronary risk. The lipid changes in this study were similar to those observed in 2 small studies of pravastatin in patients taking PIs; however, those studies were open-label and did not have a placebo control.13, 14 Those studies also were limited by standard enzymatic lipid measurements, which do not elucidate the lipoprotein changes observed with lipid-lowering interventions and can be misleading in patients with hypertriglyceridemia.17
In this study, endothelial function was evaluated with brachial artery ultrasound scanning, a well-established research technique.20, 26 FMD of the brachial artery is reproducible, correlates strongly with FMD of the coronary arteries, and is predictive of future adverse cardiovascular events.26, 27, 28, 29, 30 Impaired FMD has been described in patients taking PIs; however, the effects of lipid-lowering therapy on FMD in patients with HIV have not been investigated previously.4, 10 In this study, short-term therapy with pravastatin was associated with a small increase in FMD that did not reach statistical significance (P = .080), even after adjustment for potential confounders. In a previous study of subjects who were not infected with HIV, short-term therapy with pravastatin did lead to significant improvements in brachial artery endothelial function.15 There are several possible explanations for the less-than-expected magnitude of FMD improvement. Subjects in this study had abnormalities of several lipoprotein subfractions, characteristic of atherogenic dyslipidemia.17 These abnormalities were manifested as combined dyslipidemia, with elevations of triglyceride- and cholesterol-containing lipoproteins, in conjunction with a decreased number of large HDL particles. Pravastatin therapy reduced the concentrations of LDL particles, small LDL, and small VLDL; however, LDL particles on average remained small, and concentrations of IDL and large VLDL (including remnants) remained elevated. Small LDL and remnant lipoproteins are associated with endothelial dysfunction.31, 32, 33 In our previous investigation, VLDL and IDL explained a significant component of the variance in FMD in individuals taking HIV PIs.4 Although the overall lipid panel on pravastatin appeared less atherogenic than at baseline, it still was quite abnormal. The lipoprotein abnormalities that persisted after statin therapy most likely contributed to the residual endothelial dysfunction after statin therapy. It is unlikely that monotherapy with pravastatin will "normalize" lipoproteins and FMD in patients taking PIs who have a similar degree of dyslipidemia.
It also is possible that the endothelial dysfunction associated with use of PIs is caused by insulin resistance and that the lipoprotein abnormalities previously associated with impaired FMD are markers of insulin resistance. In an intriguing study, 6 men who were HIV-negative took indinavir for 4 weeks.10 The use of indinavir decreased insulin sensitivity and blunted methacholine-induced increases in leg blood flow, in the absence of lipid changes.10 Although lipoproteins were not measured, this preliminary observation suggests that use of PIs rapidly impairs insulin sensitivity and that insulin resistance per se may impair nitric oxide production. Insulin resistance also has been associated with increased levels of remnant lipoproteins and coronary endothelial dysfunction, suggesting that the vascular effects of insulin resistance and lipoprotein metabolism are closely related.32, 34 In the present study, an exploratory analysis identified glucose, IDL, and HDL cholesterol levels as significant predictors of change in FMD with pravastatin. These findings are consistent with previous investigations in which triglyceride-rich lipoproteins, remnant particles, HDL cholesterol and glucose levels helped explain the variance in FMD and suggest that more aggressive lipid-lowering therapy will be needed to improve endothelial function.4 Although the sample size estimates were verified with data from our laboratory and previously published sample-size nomograms, a type-II error on change in FMD still was possible.4, 19, 20 Furthermore, the long-term safety of use of pravastatin in patients taking HIV PIs needs to be established. In this and other short-term studies in patients taking HIV PIs, pravastatin has been well tolerated.13, 14
Conclusions
This is the first double-blind, placebo-controlled study of the effects of pravastatin on lipids and lipoprotein subfractions in patients taking HIV PIs. Therapy with pravastatin was associated with reductions in concentrations of atherogenic lipoproteins. In studies of patients without HIV infection, these changes decreased coronary risk. In this study, the use of pravastatin was associated with a trend toward improved endothelial function. Combinations of lipid-lowering therapies may be needed to normalize the lipoprotein abnormalities and endothelial dysfunction observed in patients taking HIV PIs.
Adverse effects and safety parameters
Significant changes in aspartate aminotransferase, alanine aminotransferase, glucose, total bilirubin, and CK levels were not observed. No subject developed an aspartate aminotransferase level >2-times ULN in this study. Two subjects had transient alanine aminotransferase increases >2-times ULN (1 receiving placebo, 1 receiving pravastatin). The average baseline CK level was 142.4 ± 32.1 U/L. It was not significantly different with placebo (279.1 ± 103.9 U/L) or pravastatin (172.6 ± 41.7 U/L, P = .347). With placebo, 1 subject had a CK increase >2-times ULN, and another subject had an asymptomatic CK increase >8-times ULN after intensive aerobic exercise and weightlifting. It decreased to <2-times ULN within 2 weeks. With pravastatin, 1 subject had an asymptomatic increase in CK >2-times ULN, and another subject had an asymptomatic increase in CK >3-times ULN. With placebo, myalgias did not develop in any subjects. With pravastatin, mild myalgia developed in 1 subject, and muscle aches characterized as "severe" developed in 2 subjects; however, they did not discontinue medical therapy and both subjects' CK levels had decreased from pretreatment values. There were no differences between the placebo and pravastatin treatments in the incidences of these adverse effects: rash, itching, vision changes, gas, heartburn, nausea, chest discomfort, headache, dizziness, cough, diarrhea, constipation, cramps, hair loss, appetite loss, dysphagia, jaundice, fatigue, flu-like symptoms, cold-like symptoms, rhinorrhea, or hospitalizations.
In the era of highly active antiretroviral therapy, the dyslipidemia observed in patients with human immunodeficiency virus (HIV) infection has become more severe and more common.1, 2, 3 Patients taking HIV protease inhibitors (PIs) frequently have hypercholesterolemia and hypertriglyceridemia, with increased concentrations of very low-density lipoproteins (VLDL) and intermediate-density lipoproteins (IDL).2, 3, 4, 5, 6 Increased triglycerides are distributed among all lipoprotein fractions and are accompanied by increased concentrations of apolipoprotein B-100 and small low-density lipoprotein (LDL) particles.4, 5, 6, 7, 8 The well-described association among the use of PIs, dyslipoproteinemia, insulin resistance, and central obesity have generated concern that the use of PIs may increase cardiovascular risk.1, 3, 9 Although not conclusive, most published studies suggest that the use of HIV PIs may be associated with an increased incidence of cardiovascular disease.3, 9 The use of HIV PIs also has been associated with endothelial dysfunction.4, 10 In a cross-sectional study, triglyceride-rich lipoproteins and cholesterol-rich remnants were predictive of endothelial dysfunction, suggesting that the dyslipoproteinemia associated with PI therapy may increase future cardiovascular risk.4
Results
Clinical parameters
Of the 20 subjects in this study, 18 were men, 16 were white, and the average age was 44.1 ± 1.6 years. Baseline subject characteristics and clinical and dietary parameters are detailed in Table I. Clinical parameters related to HIV infection and therapy are detailed in Table II. All subjects were taking HIV PIs. On average, subjects had been taking PIs for 4.2 ± 1.3 months. The most commonly used PI was ritonavir, which was used to boost serum levels of another HIV PI in all 9 subjects who were taking this medication. All subjects also were receiving nucleoside reverse transcriptase inhibitors, and some received non-nucleoside reverse transcriptase inhibitors. Most subjects had undetectable HIV ribonucleic titers (Table II).
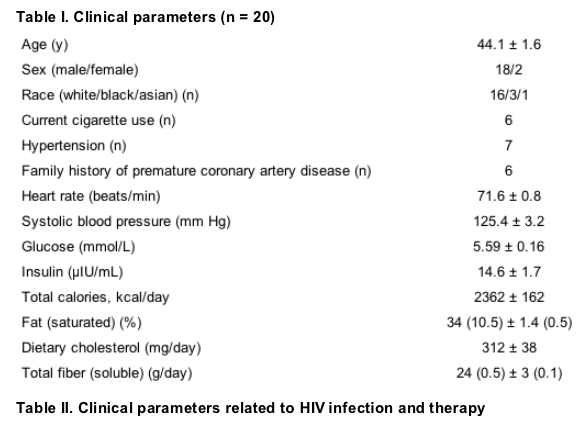
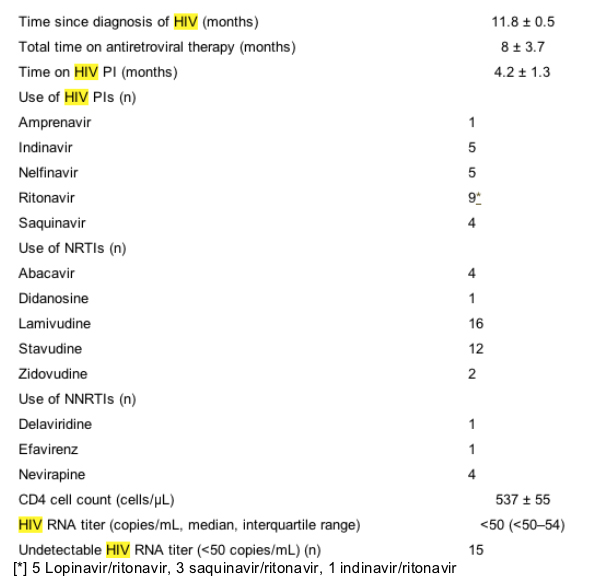
Lipid, lipoprotein, and ultrasound scanning parameters
Subjects had mild hypercholesterolemia, significant hypertriglyceridemia, and low HDL cholesterol levels. Non-HDL cholesterol levels were elevated. Six subjects had detectable chylomicrons. On average, subjects had an increased concentration of LDL particles (1756 ± 180 nmol/L), which tended to be small (19.9 ± 0.2 nm), a low concentration of large HDL particles (0.32 ± 0.06 mmol/L), and an increased concentration of large VLDL particles (4.34 ± 1.33 mmol/L). At baseline, FMD was impaired (4.5% ± 1.1%), which was indicative of endothelial dysfunction. NTGMD also was impaired slightly (14.3% ± 1.0%; Table III).
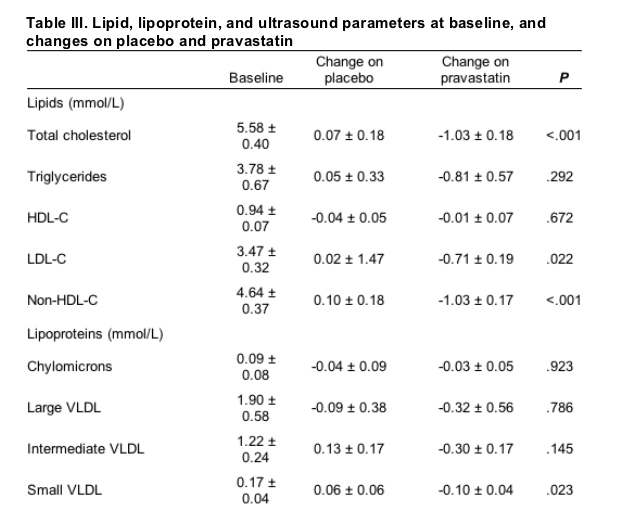
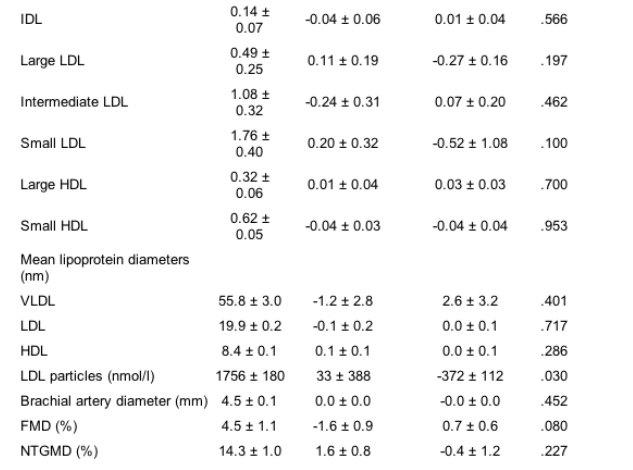
Methods
Experimental protocol and clinical parameters
The Institutional Review Board of the University of Wisconsin Medical School approved this study. All subjects provided informed consent. Consecutive patients with HIV infection of ≥6-month duration were screened for participation. Subjects were required to be undergoing a stable antiretroviral regimen that included a PI for at least 3 months. Subjects had LDL cholesterol levels >3.36 mmol/L and either triglyceride levels >3.88 mmol/L or high-density lipoprotein (HDL) cholesterol levels <1.07 mmol/L. Patients with coronary artery disease, a recent opportunistic infection, acquired immune deficiency syndrome-defining illness, or malignancy were excluded, as were patients taking anabolic steroids, corticosteroids, or lipid-lowering medications or who smoked >1 pack of cigarettes per day. Subjects were excluded when they had diabetes mellitus, hypothyroidism, aspartate aminotransferase, alanine aminotransferase, total bilirubin or creatine kinase (CK) levels >3 times the upper limit of normal (ULN), or creatinine level >176.8 μmol/L.
This was a placebo-controlled, double-blind, crossover trial. All subjects and study staff were blinded to treatment; however, the principal investigators (J.H.S., J.M.S.) were aware of the treatment schema. Participation began with a 0- to 4-week dietary stabilization period to ensure subject compliance with a low-fat, low-cholesterol, calorically appropriate diet. Dietary intake, counseling, and stabilization were coordinated by a registered dietitian with a Master's degree in nutrition (G.L.U.) using 3-day food records (Nutritionist Five, First Data Bank, San Bruno, Calif). Subjects whose diets were not consistent with the American Heart Association step 1 diet received counseling and follow-up until stabilization and compliance could be verified. Continued dietary compliance was evaluated at each visit.
After dietary stabilization, subjects entered treatment phase 1, which consisted of placebo-pravastatin therapy, 1 tablet nightly for 8 weeks. This was followed by treatment phase 2, which consisted of pravastatin (Pravachol, Bristol-Myers Squibb, Princeton, NJ), 40 mg nightly for 8 weeks. Blood tests, ultrasound scanning examinations, and interim medical history were obtained after weeks 0, 8, and 16. Every 4 weeks, subjects were contacted by telephone and encouraged to take their prescribed medications, follow a stable diet, and report adverse effects.
Measurement of lipid and lipoprotein parameters
Blood was collected into lavender-topped collection tubes and centrifuged immediately at 3000 rpm for 15 minutes. Plasma was transferred to a cryovial, refrigerated at 4 C, and shipped within 24 hours in a refrigerated box to LipoScience (Raleigh, NC) for nuclear magnetic resonance spectroscopic lipoprotein analysis.17, 18 Sixteen measured lipoprotein subclasses were combined into 10 categories: chylomicrons (>200 nm), large VLDL and chylomicron remnants (60-200 nm), intermediate VLDL (35-60 nm), small VLDL (27-35 nm), IDL (23-27 nm), large LDL (21.3-23.0 nm), intermediate LDL (19.8-21.2 nm), small LDL (18.8-19.7 nm), large HDL (8.2-13 nm), and small HDL (7.3-8.2 nm). Levels of chylomicrons and VLDL subclasses were expressed in units of triglycerides (mmol/L). LDL and HDL subclass concentrations were expressed in units of cholesterol (mmol/L). Average diameters of VLDL, LDL, and HDL particles were determined by weighing the relative mass percentage of each subclass by their mean diameters.17, 18 Nuclear magnetic resonance-derived lipid values (total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol) were determined by conversion of lipoprotein particle data to lipid concentration units on the basis of the expected amount of cholesterol and triglycerides in each particle.17
Other laboratory tests
Fasting serum glucose, aspartate aminotransferase, alanine aminotransferase, and total bilirubin levels were measured with standard enzymatic techniques on a Hitachi 747 analyzer (Roche Diagnostics, Basel, Switzerland). Fasting serum insulin level was measured with a chemiluminescent immunoassay on a Diagnostic Products Corporation Immulite 2000 analyzer (Los Angeles, Calif). CD4 cell counts were measured with flow cytometry. Plasma HIV ribonucleic acid titers were measured with b-deoxyribonucleic acid hybridization (Chiron, Emeryville, Calif).
Measurement of brachial artery ultrasound parameters
Brachial artery reactivity studies were performed in the morning on the day of phlebotomy in the fasting state. Subjects who smoked cigarettes abstained for at least 12 hours. Hyperemia was induced by inflating a tourniquet around the forearm to a suprasystolic pressure for 4.5 minutes. Flow-mediated vasodilation (FMD) of the brachial artery was measured 1 minute after cuff deflation. After a 15-minute recovery period, sublingual nitroglycerin (400 mcg) was administered. Nitroglycerin-mediated vasodilation (NTGMD) was assessed 3 minutes later. Images were obtained with a 7.5-mHz linear array ultrasound scanning transducer and Sonos 5000 Ultrasound scanning system (Philips Medical Systems, Andover, Mass). Brachial artery diameters were measured in triplicate over a 1-cm segment with digital calipers (AccessPoint 2000, Freeland Systems, Indianapolis, Ind). Measurements were performed by staff members who were blinded to subject information, including treatment phase and study date. Reliability for measurement of brachial artery diameters is 0.987, reflecting an interclass correlation coefficient across all readings and conditions.19
Statistical analysis
Parameters were described with means plus or minus standard error (SE). Comparisons in treatment phases initially were performed with paired Student t tests. Multivariate regression was used to identify predictors of FMD and predictors of change in FMD with pravastatin. Change in FMD during each treatment phase also was assessed after adjustment for between-visit differences in baseline brachial artery diameter and reactive hyperemia flow. Adverse effects, safety parameters, and dietary composition were evaluated with repeated measures analysis of variance. Changes in adverse-effect rates were evaluated with the binomial test. On the basis of published sample-size nomograms and data from our laboratory, this study had >80% power to detect a 2% change in FMD (α = 0.05).4, 19, 20
The Acquired Immunodeficiency Syndrome Clinical Trials Group guidelines for the evaluation and management of dyslipidemia in adults infected with HIV who are receiving antiretroviral therapy recommended treatment of dyslipidemia with pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor that is not metabolized by cytochrome P450 3A4, the isoenzyme system inhibited by currently available HIV PIs.3, 11 Although pravastatin has been proven to reduce first coronary events, its effects on lipoproteins in individuals taking PIs have not been described previously.12 Although modest reductions in total cholesterol, LDL cholesterol, and triglyceride levels have been observed in patients who are treated with PIs and taking pravastatin, these studies have been small and open-label and were not placebo-controlled.13, 14 Pravastatin improves endothelial function in patients with dyslipidemia; however, its arterial effects in patients with HIV infection are not known.15, 16 The objective of this study was to determine the effects of pravastatin on lipoprotein subfractions and endothelial function in patients with dyslipidemia who were receiving HIV PIs.
|
|
| |
| |
|
|
|