| |
Hi Rates of Transmitted HIV Drug Resistance in NYC: 13-24% in NYC; NNRTI Rate Increased from 5.5% to 13.4% from 2001-02 to 2003-04
|
| |
| |
"Tracking the Prevalence of Transmitted Antiretroviral Drug-Resistant HIV-1: A Decade of Experience"
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 41(4) 1 April 2006 pp 439-446
Shet, Anita MD; Berry, Leslie BS; Mohri, Hiroshi MD, PhD; Mehandru, Saurabh MD; Chung, Chris MA; Kim, Alexandria BS; Jean-Pierre, Patrick BS; Hogan, Christine MD; Simon, Viviana MD, PhD; Boden, Daniel MD; Markowitz, Martin MD
From the Aaron Diamond AIDS Research Center (an affiliate of the Rockefeller University), New York, NY.
Note from Jules Levin: at the Resistance Workshop in June there was study research finding that a sensitive genotypic resistance test could identify resistance that standard genotypic tests could not for NNRTI resistance. Over the past several years studies finding similarly have been reported at the Resistance Workshop. This raises the concern that the study presented here underestimates the real prevalence. There was controversy at the Workshop regarding the findings because using such sensitive assays in the real world is difficult because the tests are very labor intensive and expensive. I think sensitive NNRTI genotypic testing is important and would uncover higher rates of resistance. Some newly infected patients may have NNRTI resistance that is undetectable with standard genotypic tests and they might receive but not respond well to firstline NNRTI therapy. At the Resistance Workshop there was also some concern expressed about increases in NNRTI resistance in transmitted HIV.
"....In summary, the data presented indicate an increasing trend in prevalence of transmitted HIV-1 drug resistance, especially NNRTI resistance among individuals newly infected with HIV-1 in our cohort in New York City followed up over a decade. These findings have crucial implications for the selection of initial antiretroviral regimens. We believe that baseline resistance testing in newly diagnosed individuals, with a subsequently tailored antiretroviral drug regimen, contributes significantly to a successful clinical outcome and should be standard of care for all new HIV-1 infections....
"....Drug resistance in the pre-highly active antiretroviral therapy era consisted almost entirely of NRTI-resistant viruses, but after 1997, following introduction of PIs and combination therapy as standard of care, there was a shift toward increasing PI and NNRTI resistance..... Our data are consistent with previous observations from the United States7,8 and indicate that transmitted NNRTI resistance continues to rise steeply. Indeed, in 2004, approximately 1 of every 7 persons newly infected with HIV-1 was unable to use an NNRTI as part of a therapeutic regimen because of drug resistance. In acute symptomatic cases where urgent therapy was warranted, we modified our practice such that therapy was empirically initiated with a lopinavir/ritonavir-based regimen, and only after viral resistance testing results indicating wild-type virus was therapy stabilized to an efavirenz-based regimen.... The prevalence of transmitted MDR HIV-1 among newly infected individuals remains a cause for concern. Other similar homogenous populations in HIV-1 "high-prevalence" areas in the United States report rates of MDR among white homosexual men as high as 10.2% to 13.2%.7,8 A lower prevalence of MDR transmission in the United States among antiretroviral-naive, but not necessarily "recently infected," patients (1.3% during the period 1997-2001) was reported among a geographically and ethnically diverse population who exhibited a diverse range of HIV-1 risk behaviors.17 Unlike acquired drug resistance in chronically infected, antiretroviral-experienced individuals,27,28 most transmitted drug-resistant mutations are generally persistent over time in the absence of drug selective pressure..."
Summary: Transmitted resistance to antiretroviral drugs in acute and early HIV-1 infection has been well documented, although overall trends vary depending on geography and cohort characteristics. To describe the changing pattern of transmitted drug-resistant HIV-1 in a well-defined cohort in New York City, a total of 361 patients with acute or recent HIV-1 infection were prospectively studied over a decade (1995-2004) with respect to HIV-1 genotypes and longitudinal T-cell subsets and HIV-1 RNA levels.
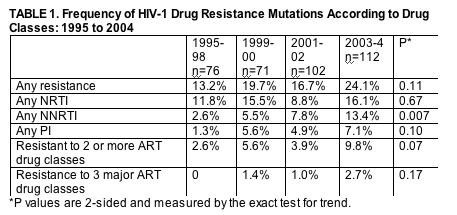
The prevalence of overall transmitted resistance changed from 13.2% to 24.1% (P = 0.11) during the periods 1995 to 1998 and 2003 to 2004.
Nonnucleoside reverse transcriptase inhibitor resistance prevalence increased significantly from 2.6% to 13.4% (P = 0.007) during the same periods, whereas prevalence of multidrug-resistant virus shifted from 2.6% to 9.8% (P = 0.07) but did not achieve statistical significance.
Three of the 11 subjects with MDR virus during 2003-2004 had viral variants with resistance to all 3 major classes of antiretroviral drugs.
A comparable immunologic and virologic response of appropriately treated individuals was observed regardless of viral drug susceptibility status, suggesting that initial combination therapy guided by baseline resistance testing in the case of acute and early infection may result in a favorable treatment response even in the case of a drug-resistant virus. These data have important implications for selection of empiric first-line regimens for treatment of acutely infected antiretroviral-naive individuals and reinforce the need for baseline resistance testing in acute and early HIV-1 infection.
Individuals newly diagnosed with acute or recent HIV-1 infection were screened at the Aaron Diamond AIDS Research Center, Rockefeller University Hospital, New York. All subjects were treatment-naive and infected with HIV-1 within the past 12 months and were either self-referred or recruited through community referrals. Eligible subjects were prospectively enrolled between July 1995 and December 2004.
Background
The occurrence of transmitted HIV-1 drug resistance is an evolutionary process that proceeds on temporal, environmental, and genomic scales. Rapid viral turnover1 and viral genetic variability continue to shape emerging trends in HIV-1 drug resistance. Recent epidemiological reports on transmitted drug resistance show conflicting trends, with some studies demonstrating a decline in prevalence2-6 and others revealing an increase in the transmission of drug-resistant HIV-1.7-10 Transmission of multidrug-resistant (MDR) HIV-1 (defined as viral variants with resistance to 2 or more classes of antiretroviral drugs) is also a major concern in primary HIV-1 infection.11 Some reports do not indicate increasing transmission of MDR HIV-1, suggesting that MDR variants may have impaired fitness and may be less efficiently transmitted.12 However, there is growing recognition that initial fitness impairment that results from emergence of certain resistance mutations can be improved by the accumulation of secondary mutations, occasionally restoring viral fitness to levels that may surpass that of the parental wild-type virus.13 Thus, temporal trends and clinical fitness of MDR HIV-1 continue to remain as topics of active debate. In this report, we summarize the changing epidemiology of transmitted HIV-1 drug resistance within this well-defined cohort in New York City during the past decade, with emphasis on the years 2003 and 2004.
DISCUSSION
In the present era of potent combination antiretroviral therapy, cohort studies indicate that the prevalence of transmitted drug-resistant HIV-1 ranges from 3.6% to 27.4% in North America.6-8,17 Primary transmission of drug-resistant virus increased steadily since 1995 and peaked in 1999 to 2000, after which an overall slower rise has been reported.7-10 Studies from Europe,2,3,5 Australia,4 and Canada6 suggested a significant drop in primary drug-resistant HIV-1 transmission after 2000, relative to the period 1996 to 2000. Data from our cohort similarly demonstrated a slight decline in prevalence of transmitted drug resistance from 19.7% (pre-2000) to 16.7% (post-2000); subsequently, however, prevalence increased to 24.1% in 2003-2004. Within the United States, other reports also showed a similar slight fall in transmitted drug resistance around 2000,8,18 followed by the occurrence of a second peak in 2001.8 The modest decline in transmitted drug resistance prevalence between 2000 and 2001 seen within our cohort as well as reported by other investigators8,18 may be the result of chance fluctuation or possibly reflective of better control of virologic outcome among potential transmitters brought about by widespread use of potent antiretroviral therapy and lower pill burden leading to improved adherence. Nonetheless, the increasing prevalence in the United States after the year 2000 is likely a consequence of viral and host factors. The trends observed within this cohort are likely multifaceted and may reflect increased initiation of highly active antiretroviral therapy during early infection, increased use of treatment interruptions, or increased risk behavior in individuals with drug resistance or chance fluctuations. Use of stimulants such as methamphetamines has been reported in certain high-risk groups19 and thought to be associated with a rising incidence of sexually transmitted disease, including HIV-1.20-22 Viral factors include the accumulation of compensatory mutations within and outside the pol region of the HIV-1 genome that may increase replication fitness and infectivity of resistant mutants.23,24 These evolutionary changes may steer the stochastic odds during transmission in favor of selection of the drug-resistant variant, thus promoting transmission of drug resistance.23,24
Drug resistance in the pre-highly active antiretroviral therapy era consisted almost entirely of NRTI-resistant viruses, but after 1997, following introduction of PIs and combination therapy as standard of care, there was a shift toward increasing PI and NNRTI resistance. However, since 2000, the picture was dominated by a sharp increase in transmitted NNRTI resistance.8,9 The mechanism of NNRTI resistance involves reduced drug-binding affinity, which is mediated by single-nucleotide changes in the NNRTI-binding site of viral RT. Because this site is situated outside the active site of HIV-1 RT, these mutations do not interfere with enzymatic activity, and therefore, viral fitness is less likely to be compromised. Previous studies have clearly shown that common NNRTI mutations, K103N, G190A,23,25 and Y181C,26 have minimal impact on viral fitness. Previously, our laboratory has shown that in vitro infectivity of patient-derived drug-resistant HIV-1 isolates was significantly higher than that of drug-susceptible isolates and that both groups displayed similar growth kinetics.24 Interestingly, the rising trend in NNRTI resistance has not been consistently reported outside the United States and may be a reflection of geographical variability of transmission and prescribing patterns. Our data are consistent with previous observations from the United States7,8 and indicate that transmitted NNRTI resistance continues to rise steeply. Indeed, in 2004, approximately 1 of every 7 persons newly infected with HIV-1 was unable to use an NNRTI as part of a therapeutic regimen because of drug resistance. In acute symptomatic cases where urgent therapy was warranted, we modified our practice such that therapy was empirically initiated with a lopinavir/ritonavir-based regimen, and only after viral resistance testing results indicating wild-type virus was therapy stabilized to an efavirenz-based regimen.
The prevalence of transmitted MDR HIV-1 among newly infected individuals remains a cause for concern. Other similar homogenous populations in HIV-1 "high-prevalence" areas in the United States report rates of MDR among white homosexual men as high as 10.2% to 13.2%.7,8 A lower prevalence of MDR transmission in the United States among antiretroviral-naive, but not necessarily "recently infected," patients (1.3% during the period 1997-2001) was reported among a geographically and ethnically diverse population who exhibited a diverse range of HIV-1 risk behaviors.17 Unlike acquired drug resistance in chronically infected, antiretroviral-experienced individuals,27,28 most transmitted drug-resistant mutations are generally persistent over time in the absence of drug selective pressure29-31 and are not associated with reduced replication capacity.32 We have shown that, even after adjusting for duration of infection, baseline HIV-1 RNA levels and CD4+ counts were similar among individuals with drug-resistant and drug-susceptible viruses, implying that in vivo fitness of these variants may becomparable.
It is notable that, among recently infected patients who were able to identify their source partners, a considerable number had acquired HIV-1 from other acute or recently infected partners rather than from individuals with long-standing infection who were failing therapy, a pattern that is illustrated in the phylogenetic tree of viral isolates from patients from 2003 to 2004 (Fig. 1). Whereas most of the individuals with acute retroviral symptoms seeking medical attention received appropriate HIV-1 testing, approximately a quarter of the individuals were either not tested for HIV-1 at all or were offered the HIV-1 antibody test alone (which is inadequate during the seronegative stage), thus resulting in several instances of missed diagnoses. The seroconversion period being associated with high viremia is a highly infectious stage, making it imperative that both the HIV-1 antibody and HIV-1 RNA viral load tests be done while entertaining a diagnosis of HIV-1. Early diagnosis of HIV-1 also presents the opportunity to consider initiation of early therapy and counseling, whose benefits are numerous, and include most importantly, from a public health perspective, decreased HIV-1 transmission.33,34
It has been suggested previously that efficacy of current antiretroviral drug regimens and benefits of early therapy may be limited by the transmission of drug-resistant HIV-1.6,7 We have shown that guided antiretroviral therapy based on drug resistance testing can abrogate this limitation. Among those from our 2003 to 2004 cohort who began potent antiretroviral therapy, the majority (77%) received a regimen containing NRTI and NNRTI, whereas 23% received a boosted PI-containing regimen. In almost all cases, therapy was begun after results from resistance testing became available. Emergent empiric therapy was begun on 5% of the subjects; however, their drug regimen was optimized in less than 2 weeks, as soon as their viral genotyping results were known. Thus, all of the subjects included in this treatment response analysis were taking active antiretroviral medications, which likely resulted in complete suppression of HIV-1 replication irrespective of drug resistance mutations. Individuals with drug-resistant and wild-type HIV-1 were able to suppress their viral burden within 114 days after starting therapy at a similar rate of decline, with comparable CD4+ lymphocyte count recovery. These data demonstrate that drug-resistant HIV-1 responds to appropriate therapy in a manner similar to that of wild-type HIV-1 and are in contrast with previous findings that drug-resistant HIV-1 responds poorly to antiretroviral therapy.7,8 A key difference is the consistent use of baseline genotypic testing to guide therapy in this report, which was not reported in previously published reports.7,8 Other factors may be related to improved adherence caused by use of once-daily dosing of NRTIs and NNRTIs and use of boosted PIs. The 3 individuals harboring 3-class MDR virus were not treated and hence not included in this analysis. Despite these limitations, our observations suggest that the choice of an appropriate initial antiretroviral regimen based on resistance testing is likely to contribute to the success of the treatment response.
The limitations of this study include the observational design of the study, which resulted in varying numbers of subjects recruited at the specified periods and nonuniformity in comparing data across these periods. However, based on our previously published data9,10 and information described in the present study, it may be seen that the demographic and clinical characteristics of the subjects have remained relatively homogenous over the past decade. Other limitations include the potential for bias because individuals in this study may not be representative of populations from other demographic or geographic areas. Although the lack of a control group undergoing no baseline resistance testing may also be viewed as a study limitation, the use of pretreatment resistance testing in newly infected, antiretroviral-naive patients is widely accepted as standard of care, and instituting a control group would have been considered unwarranted.
In summary, the data presented indicate an increasing trend in prevalence of transmitted HIV-1 drug resistance, especially NNRTI resistance among individuals newly infected with HIV-1 in our cohort in New York City followed up over a decade. These findings have crucial implications for the selection of initial antiretroviral regimens. We believe that baseline resistance testing in newly diagnosed individuals, with a subsequently tailored antiretroviral drug regimen, contributes significantly to a successful clinical outcome and should be standard of care for all new HIV-1 infections.
RESULTS
Subject Characteristics
A total of 361 eligible individuals with acute and recent HIV-1 infection were enrolled at the Rockefeller Hospital Clinic between July 1995 and December 2004. Among the 116 newly infected individuals who were enrolled during the period from 2003 to 2004, the viral sequence from one patient could not be successfully amplified for genotyping, and 3 subjects had spontaneously controlled their virus to undetectable levels at baseline testing. Thus, 112 eligible individuals from 2003 to 2004 with new-onset HIV-1 infection were available for detailed analysis. These subjects were predominantly male (98%) and reported a history of having sex with men (97%). Mean age was 34.6 years (SD, 7.9 years; range, 19-56 years). Non-Hispanic whites constituted 71% of the subjects, and the rest consisted of 15% Hispanic, 9% African American, and 5% Asian. Baseline mean plasma HIV-1 RNA level was 5.2 log copies/mL (SD, 1.03 log copies/mL; range, 2.59-7.51 log copies/mL), mean CD4+ T-lymphocyte count was 455 cells/μL (SD, 199.7 cells/μL; range, 111-1083 cells/μL), and mean CD4+/CD8 ratio was 0.57 (SD, 0.39; range, 0.07-1.81). Baseline drug resistance analysis was performed before initiation of therapy at a mean of 58 days after the estimated date of HIV-1 infection. Approximately 10% of the subjects were still seronegative for HIV-1 or had an evolving HIV-1 Western blot at the time of diagnosis and baseline testing and were classified as having "acute" infection; 70% of the subjects were recently infected within 6 months of diagnosis, and the remaining 20% were infected between 6 and 12 months from the time of diagnosis.
Drug Resistance Trends
The prevalence of transmitted resistance during the period 2003 to 2004 was 24.1% (95% confidence interval [CI], 16.5%-33.1%), which shifted from 13.2% (95% CI, 6.5%-22.9%) during the period from 1995 to 1998 (P = 0.11; Table 1). The proportion with individual antiretroviral drug class resistance varied over the past decade; trends which did not achieve significance were NRTI resistance mutations, which varied from 11.8% (95% CI, 5.6%-21.3%) during the period from 1995 to 1998 to 16.1% (95% CI, 9.8%-24.2%) during 2003-2004 (P = 0.67), and protease inhibitor (PI) resistance-associated mutations, which varied from 1.3% (95% CI, 0.03%-6.1%) to 7.1% (95% CI, 3.1%-13.6%) during the same 2 periods (P = 0.10). Of note, prevalence of nonnucleoside RT inhibitor (NNRTI) resistance increased significantly from 2.6% (95% CI, 0.3%-6.2%) in 1995-1998 to 13.4% (95% CI, 9.1%-23.2%) during 2003-2004 (P = 0.007). Prevalence of transmitted MDR virus also shifted from 2.6% (95% CI, 1.7%-3.8%) in 1995-1998 to 9.8% (95% CI, 5.0%-16.9%) during the period 2003-2004 (P = 0.07), although the numbers were too small to achieve statistical significance. Three of the 11 subjects with MDR virus during 2003-2004 had viral variants with resistance to all 3 major classes of antiretroviral drugs. Temporal trends among individual resistance mutations are shown in Table 2.
Phylogenetic analysis of HIV-1 RT and PR sequences revealed independent segregation of most sequences from each other and from specific laboratory strains, pNL4.3, BH5, and YU.2 (Fig. 1). Certain transmission events known by history between partner pairs were revealed within the tree as displaying a close degree of homology between the respective sequences (J18 and D5, 99.1% homology; T2 and D13, 99.7% homology; A3 and H2, 99.3% homology; D2 and K4, 99.9% homology; and X1 and R4, 99.8 % homology). This finding suggested that, in some cases, HIV-1 transmission occurred with an undiagnosed, recently infected individual acting as the source.
Baseline Clinical Correlates
Baseline clinical and virologic correlates between subjects who had acquired wild-type virus (n = 85) and those who had acquired resistant virus (n = 27) during the period from 2003 to 2004 were similar. Median age and racial and ethnicity patterns were similar between the 2 groups. There was no statistically significant difference in mean baseline plasma HIV-1 RNA levels between individuals with wild-type virus (mean, 5.2 log copies/mL; 95% CI, 5.0-5.4 log copies/mL) and those with resistant virus (mean, 5.4 log copies/mL; 95% CI, 5.1-5.8 log copies/mL; P = 0.36) even after adjusting for duration of infection. Similarly, comparison of mean baseline CD4+ T-lymphocyte count also showed no statistically significant difference between individuals with wild-type virus (mean, 474 cells/μL; 95% CI, 432-518 cells/μL) and those with drug-resistant virus (mean, 398 cells/μL; 95% CI, 323-474 cells/μL; P = 0.07).
Virologic and Immunologic Response to Therapy
Of the 112 newly infected treatment-naive subjects enrolled between January 2003 and December 2004, 73 (65.2%) were initiated on an appropriate and potent antiretroviral regimen16 according to their baseline viral resistance testing. Eleven subjects (9.8%) were not included in this analysis because of unavailability of complete data through the period of analysis (6 subjects were unable to keep study visit appointments, and 5 subjects were followed up at other sites). Therapy was deferred in the remaining 28 subjects (25%; 25 showed spontaneous control of viral replication resulting in low plasma HIV-1 viral load and a CD4+ count of more than 350 cells/μL, and 3 subjects had MDR virus resistant to all 3 major classes of antiretroviral drugs). For the purpose of this analysis, subjects were followed up until they achieved undetectable HIV-1 RNA levels. Demographic and clinical characteristics among subjects with wild-type and resistant viruses included in this analysis, as well as the average number of antiretroviral drugs used in their regimens, were comparable.
The median time to viral suppression to an undetectable level (<50 copies/mL) among subjects with wild-type virus was 112 days (95% CI, 98.3-125.7 days), and that among subjects with drug-resistant virus was 114 days (95% CI, 108.4-119.6 days). Thus, there was no statistically significant difference in the time to viral suppression between the 2 groups (P = 0.83; Fig. 2). At time of viral suppression, the median CD4+ T-cell counts among subjects with wild-type and those with resistant virus were 613 and 620 cells/μL (P = 1.0), respectively; CD8+ T-cell counts were 682 and 637 cells/μL, respectively; and CD4+/CD8+ ratios were 0.91 and 1.01, respectively. The rate of decline in plasma HIV-1 RNA levels between the 2 groups within the first 20 weeks after initiation of therapy was similar (P = 0.66; FIG. 3) No significant difference in the rates of CD4+ T-lymphocyte count increase (P = 0.62) or CD8+ T-lymphocyte count decrease (P = 0.57) as a response to initiation of therapy was observed between the 2 groups of patients.
METHODS
Study Population
Individuals newly diagnosed with acute or recent HIV-1 infection were screened at the Aaron Diamond AIDS Research Center, Rockefeller University Hospital, New York. All subjects were treatment-naive and infected with HIV-1 within the past 12 months and were either self-referred or recruited through community referrals. Eligible subjects were prospectively enrolled between July 1995 and December 2004. Eligibility criteria included documentation of "acute" (defined by the presence of positive HIV-1 RNA levels and absent or evolving HIV-1 enzyme immunoassay [EIA] or Western blot with subsequently documented HIV seroconversion) or "recent" (defined by the presence of a positive HIV-1 EIA or Western blot, and either [a] a documented negative HIV-1 EIA within the previous 12 months or [b] recent HIV-1 infection evidenced by a negative reduced-sensitivity ["detuned"] EIA.14) HIV-1 infection. A detailed sexual exposure history and presence of typical symptoms of acute seroconversion illness in conjunction with serologic and virologic laboratory data were used to estimate the timing of HIV infection. A distinct history of acute retroviral syndrome after high-risk sexual activity or needle use was reported by 82% of the subjects. Among them, the date of HIV-1 infection was either reported or estimated as 14 days before the date of onset of acute retroviral symptoms. Among the remaining 18% of subjects, the date of HIV-1 infection was estimated as 30 days before a negative or indeterminate HIV-1 Western blot, or the midpoint between the last documented negative HIV-1 EIA and the first positive HIV-1 EIA or HIV-1 RNA level. Subjects enrolled between 1995 and 2000 were previously reported9,10 and have been included here for analyses of temporal trends of drug resistance prevalence.
Analyses of Resistance-Associated Mutations
Pretreatment plasma viral RNA was extracted using a QIAamp viral extraction kit (Qiagen, Valencia, CA). Population-based nucleotide sequence analysis of the HIV-1 protease (PR) gene and reverse transcriptase (RT) gene was performed using the TRUGENE HIV-1 G9 genotyping kit (Bayer Diagnostics, Tarrytown, NY) and GeneObjects 3.2 software. Between 1995 and 1999, genotypic analysis was performed using an in-house nested polymerase chain reaction followed by direct sequencing of PR and RT genes on an automated ABI sequencer.10 Amino acid substitutions in the PR and RT genes were characterized as drug resistance mutations using the updated IAS-USA Consensus guidelines.15 Nucleotide substitutions resulting in RT T215 mutations (T215S/C/D/E/N) that are intermediates of the well-characterized nucleoside RT inhibitor (NRTI) resistance-conferring mutation T215Y/F were included as major drug resistance mutations. PR-RT sequences were aligned using MegAlign 5.08 (DNASTAR Inc) and a phylogenetic tree constructed using ClustalX 1.83 and TreeView 1.6.6, 2001, to investigate whether there was laboratory strain contamination and to confirm linked viruses within the cohort.
Clinical Correlates
Plasma HIV-1 RNA level was measured using the Roche HIV-1 Amplicor version 1.5 (Roche Diagnostics, Pleasanton, CA), and CD4+ T-lymphocyte cell counts were measured using flow cytometry.
Response to Treatment
Virologic and immunologic response to therapy was evaluated among those subjects enrolled between January 2003 and December 2004 who began receiving a potent antiretroviral regimen designed according to their individual viral susceptibility profile. HIV-1 RNA levels and CD4+ and CD8+ T-lymphocyte counts were available every week for the first 4 weeks and every 4 to 6 weeks thereafter. The time to virological suppression was defined as the duration between the first day of antiretroviral therapy initiation to the first of 2 consecutive plasma HIV-1 RNA levels of less than 50 copies/mL collected at least 7 days apart.
Statistical Methods
Data from subjects were categorized according to time of enrollment and baseline testing. For convenience, 4 time periods were used to cover the 10-year period of the study: July 1995 to December 1998, January 1999 to December 2000, January 2001 to December 2002, and January 2003 to December 2004. Differences in continuous variables were assessed using the nonparametric Kruskal-Wallis test. Baseline clinical correlates were compared using linear regression after adjusting for duration of infection. Temporal changes in drug resistance and individual mutation frequencies were analyzed using the exact test for trend. Time to viral suppression was evaluated with Kaplan-Meier survival analysis. Virologic and immunologic responses were assessed using repeated-measurements analysis. All statistical tests were 2-sided (P < 0.1). Statistical analyses were performed using SPSS version 10.0 for Windows (Chicago, Ill).
|
|
| |
| |
|
|
|