| |
Coinfected with Normal ALT Have HCV Disease
|
| |
| |
"Liver Biopsy Findings for HIV-Infected Patients with Chronic Hepatitis C and Persistently Normal Levels of Alanine Aminotransferase"
Clinical Infectious Diseases Sept 1, 2006;43:640-644
Matilde Sanchez-Conde,1 Juan Berenguer,1 Pilar Miralles,1 Federico Alvarez,2 Juan Carlos Lopez,1 Jaime Cosin,1 Catalan Pilar,3 Margarita Ramirez,1 Isabel Gutierrez,1 and Emilio Alvarez2
1Infectious Diseases-HIV Unit, 2Department of Pathology, and 3Clinical Microbiology Service, Hospital Gregorio Maranon, Madrid, Spain
"....subgroup may have active liver disease that is difficult to predict on the basis of clinical or biochemical parameters only. Our data suggest that liver biopsy should be performed for HIV-HCV-coinfected patients with persistenly normal ALT levels to determine the extent of liver fibrosis, and, consequently, to assess suitability for treatment...."
Abstract
Background. Severe liver fibrosis is common in patients coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) who have a high alanine aminotransferase (ALT) level. However, little is known about the frequency, liver biopsy findings, and significance of a persistently normal ALT level in coinfected patients.
Methods. We analyzed clinical data and histological findings for 256 patients coinfected with HIV and HCV, 24 (9.4%) of whom had an ALT level within the normal range on 2 separate occasions within a 6-month period.
Results.
The proportion of patients demonstrating advanced stages of fibrosis (F3 and F4) was 78 (33.7%) of 232 patients in the high ALT level group, compared with 0 (0%) of 24 patients in the persistently normal ALT level group (P < .001).
Among patients with persistently normal ALT levels, 23 (96%) had any grade of fibrosis, and 7 (29%) had stage F2 of fibrosis.
No differences were found between both groups with respect to age, sex, HIV transmission category, Centers for Disease Control and Prevention clinical category, CD4+ cell count (both nadir and baseline values), type of antiretroviral therapy, years since onset of HCV infection, alcohol use, or HCV load.
However, the proportion of patients infected with HCV genotype 3 was significantly higher among patients with high ALT levels than in patients with persistently normal ALT levels (61 [26.9%] of 232 patients vs. 1 [4.2%] of 24 patients; P = .04).
....Significant differences were also found in activity between patients in both groups. The proportion of patients with advanced activity (grades A3 and A4) was 42 (18.1%) of 232 patients in the high ALT level group versus 2 (8.3%) of 24 patients in the persistently normal ALT level group (P = .03). The proportion of patients with steatosis was 130 (57.5%) of 226 patients in the high ALT level group versus 12 (54.5%) of 22 patients in the persistently normal ALT level group (P = .07), and the proportion of patients with esteatosis >5% was 27 (11.9%) of 226 patients in the high ALT level group versus 0 (0.0%) of 22 patients in the persistently normal ALT level group (P = 2.9...
Conclusions. Histological abnormalities are significantly milder in patients coinfected with HIV and HCV who have persistently normal ALT levels than those found in patients with high ALT levels. However, a subgroup of patients with persistently normal ALT levels may have significant cases of fibrosis. Liver biopsy may be recommendable in patients coinfected with HIV and HCV who have persistently normal ALT levels, to determine the extent of liver fibrosis and, consequently, to assess suitability for treatment.
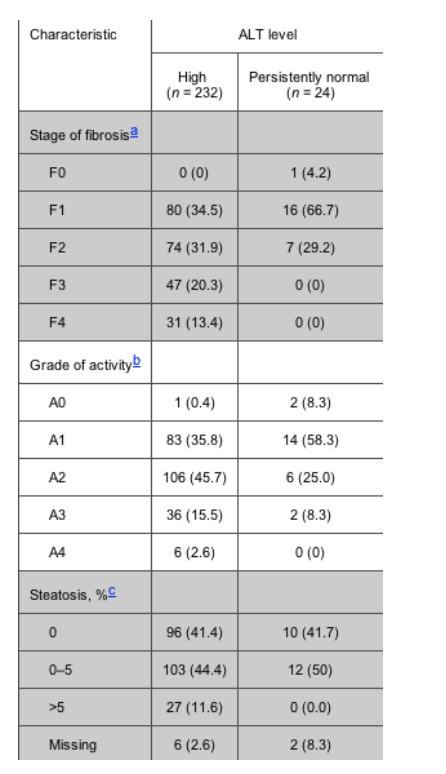
Table 2. Stage of liver fibrosis and grade of activity in patients with high alanine aminotransferase (ALT) levels and patients with persistently normal ALT levels, according to the Batts and Ludwing criteria.
Background
Case series and natural history studies have shown that disease progression in patients with an HCV infection and a persistently normal serum alanine aminotransferase (ALT) level is minimal, and most published studies of histological lesions in this population group have shown that most patients have no or minimal activity and/or fibrosis [1-7].
Coinfection with HIV and HCV is a common problem, given that both viruses share routes of transmission. It is calculated that the prevalence of HCV infection is about 33% among HIV-infected persons, a percentage that is as high as 60%-90% among injection drug users and 85% among hemophiliacs [8]. There is much evidence showing that immunosuppression secondary to HIV infection hastens the natural history of chronic hepatitis C infection, resulting in a greater frequency and speed of progression to cirrhosis, hepatic decompensation, hepatocellular carcinoma, and death in coinfected patients than in HCV-monoinfected patients [9-14].
Severe liver fibrosis is common among patients coinfected with HIV and HCV who have high ALT levels [15]. However, little is known about the frequency, liver biopsy findings, and significance of persistently normal ALT levels in coinfected patients. We studied the liver biopsies of HIV-HCV-coinfected patients with persistently normal ALT levels and compared them with those of HIV-HCV-coinfected patients with high ALT levels.
RESULTS
During the study period, we performed a liver biopsy on 256 consecutive HIV-HCV-coinfected patients, 24 (9.4%) of whom had persistently normal ALT levels. Characteristics of patients with high ALT levels and patients with persistently normal ALT levels are shown in table 1. More than two-thirds of all patients were male, and the median age was 39 years. Almost 80% of patients were determined to be in the injection drug category for HIV transmission. The median duration of HCV infection was 20 years, and approximately one-half of the patients had a history of high alcohol intake. Less than one-third of patients had prior AIDS-defining conditions. The median nadir CD4+ T cell count was >200 cells/mL, the median baseline CD4+ T cell count was 500 cells/mL, and two-thirds of patients had an HIV load below the lower limit of quantification (50 copies/mL).
When clinical and laboratory parameters for both groups were compared, no differences were found with respect to age, sex, HIV transmission category, Centers for Disease Control and Prevention clinical category, CD4+ cell count (both nadir and baseline values), type of HAART, years since onset of HCV infection, alcohol use, and HCV load. However, significant differences were found with respect to HCV genotype. The proportion of patients infected with HCV genotype 3 was significantly higher among patients with high ALT levels than among patients with persistently normal ALT levels (61 [26.9%] of 232 patients vs. 1 [4.2%] of 24 patients; P = .04).
Estimates of liver fibrosis stage, the degree of activity, and the percentage of hepatocytes containing visible macrovesicular steatosis are shown intable 2. There were striking differences in fibrosis stage between patients with high ALT levels and patients with persistently normal ALT levels. The proportion of patients with advanced stages of fibrosis (F3 and F4) was 78 (33.7%) of 232 patients in the high ALT level group versus 0 (0%) of 24 patients in the persistently normal ALT level group (P < .001). It is worth noting that, although neither the F3 stage of fibrosis nor the F4 stage of fibrosis was found among patients with persistently normal ALT levels, 23 patients (96%) had any grade of fibrosis, and 7 patients (29%) had F2 fibrosis.
Significant differences were also found in activity between patients in both groups. The proportion of patients with advanced activity (grades A3 and A4) was 42 (18.1%) of 232 patients in the high ALT level group versus 2 (8.3%) of 24 patients in the persistently normal ALT level group (P = .03). The proportion of patients with steatosis was 130 (57.5%) of 226 patients in the high ALT level group versus 12 (54.5%) of 22 patients in the persistently normal ALT level group (P = .07), and the proportion of patients with esteatosis >5% was 27 (11.9%) of 226 patients in the high ALT level group versus 0 (0.0%) of 22 patients in the persistently normal ALT level group (P = 2.9).
DISCUSSION
A persistently normal ALT level in patients with chronic hepatitis C traditionally has been defined as the presence of 3 consecutive determinations within the normal range during a 6-month period [18, 19]. Using this definition, 25%-40% of patients with chronic HCV infection have persistently normal ALT levels [20, 21], although little is known about the frequency and significance of persistently normal ALT levels in HIV-HCV coinfected patients. A retrospective study performed in a French institution found that 28.5% of 137 HIV-infected patients with PCR-confirmed HCV infection had persistently normal ALT levels [22]. Two cross-sectional studies performed in Spain found that 24% and 31% of HIV-HCV-coinfected patients had persistently normal ALT levels [23].
Most published studies of liver biopsies of HCV-infected patients with persistently normal ALT levels have shown that the majority have no or minimal-stage fibrosis [1-7]. However, fibrosis of a significant extent has been reported in 5%-30% of cases, depending on the inclusion criteria and the length of baseline follow-up [24]. In our study, we found that histological abnormalities were significantly milder in HIV-HCV-coinfected patients with persistently normal ALT levels than those in patients with high ALT levels. An advanced stage of fibrosis (F3 and F4) was observed in the liver biopsies of approximately one-third of the patients with high ALT levels versus none in patients with persistently normal ALT levels. Nevertheless, it is worth noting that, despite this finding, we observed a significant stage of fibrosis (F2) in 29% of HIV-HCV-coinfected patients with persistently normal ALT levels. This figure agrees with the results published in a recent article in which 25% of the liver biopsies obtained from 24 HIV-HCV-coinfected patients who had persistently normal ALT levels demonstrated a stage of fibrosis >F1 [25].
The reason why serum ALT concentrations are normal in some patients and high in others is not well understood. In our study, we did not identify any host or treatment-related factor to be significantly different between patients with persistently normal ALT levels and patients with high ALT levels. However, 1 virus-related factor-genotype-was significantly different between the groups. HCV genotype 3 was significantly more common among patients with high ALT levels than among patients with persistently normal ALT levels. The results of studies involving HCV-monoinfected patients that have analyzed the association of HCV genotypes with persistently normal ALT levels differ widely. Some have found that patients with genotype 3 are more likely to have normal ALT levels [26], some have found that patients with genotype 2 are more likely to be at lower risk of having abnormal ALT levels [27], and others have found no relationship between persistently normal ALT levels and viral genotype [28]. In any case, it is important to mention that several differential characteristics of HCV genotype 3 have been appreciated. For example, some studies have shown that patients infected with HCV genotype 3 may show faster progression of liver fibrosis, often accompanied by liver steatosis [29]. In the same way, a recent article [30] has shown that, independent of other clinical variables, HIV-infected patients coinfected with HCV genotype 3 were at a higher risk for relevant hepatotoxicity after receipt of HAART.
One potential limitation of our study is the duration of the observational period and the number of ALT measurements used to identify patients with persistently normal ALT levels. This has been a matter of controversy, mainly because ALT reactivation may occur in 15%-20% of these patients at further follow-up visits, thereby establishing the need for ALT monitoring in all patients initially defined as being HCV-infected patients with persistently normal ALT levels [24]. In any case, from a practical point of view, a 6-month baseline with at least 2-3 separate ALT determinations is still considered to be the standard for defining this group of patients.
Despite their shortcomings, we believe that the implications of our findings are relevant for practice, because they clearly show that, although the majority of HIV-HCV-coinfected patients with normal ALT levels have minimal liver damage, a subgroup may have active liver disease that is difficult to predict on the basis of clinical or biochemical parameters only. Our data suggest that liver biopsy should be performed for HIV-HCV-coinfected patients with persistenly normal ALT levels to determine the extent of liver fibrosis, and, consequently, to assess suitability for treatment.
METHODS
The study population was selected from the HIV outpatient clinic at the Hospital Gregorio Maranon (Madrid, Spain). Starting in September 2000, when IFN- and ribavirin therapy was approved for the treatment of chronic hepatitis C in HIV-infected patients in our institution, we prospectively performed a liver biopsy on HIV-HCV-coinfected patients who were potential candidates for this therapy. Potential candidates included HIV-infected patients with HCV infection confirmed by a qualitative HCV RNA assay, negative results of testing for hepatitis B surface antigen, a CD4+ lymphocyte count >200 cells/L, receipt of a stable antiretroviral therapy or no need for antiretroviral therapy, and absence of active opportunistic infection, active drug or alcohol addiction, and contraindications for IFN- and ribavirin therapy. From September 2000 to January 2002, we also required high ALT serum levels, and from February 2002, we included patients with persistently normal ALT levels (i.e., levels found to be within the normal range on 2 separate occasions at least 1 month apart during the previous 6 months). The last patient included in this report underwent biopsy in February 2005.
On the day of the biopsy, a computerized case report form was completed for each patient. The following information was obtained from the medical record: age, sex, risk category, Centers for Disease Control and Prevention clinical category, prior antiretroviral therapy (if any), complete blood counts, liver panel, basic metabolic panel, coagulation test results, plasma HIV RNA levels, and CD4+ T cell counts (nadir and baseline values). Duration of HCV infection for injection drug users was calculated from the first year in which needles were unsafely shared. Duration of HCV infection was considered to be unknown for subjects infected by means of sexual contact. Patients were also asked about their alcohol use. We considered consumption of >50 g of alcohol per day for >12 months to be high intake.
HIV infection was documented in all patients by ELISA and a Western blot assay. All patients tested positive for specific HCV antibodies and had serum levels of HCV RNA detectable by PCR. HCV load was measured by PCR (Cobas Amplicor HCV Monitor Test; Roche), and the results were reported in IU/mL. HCV genotype was determined by hybridization of biotin-labeled PCR products to oligonucleotide probes bound to nitrocellulose membrane strips (INNO-LiPA HCV II; Innogenetics).
Liver biopsy was performed on an outpatient basis in accordance with the recommendations of the Patient Care Committee of the American Gastroenterological Association [16]. Ultrasound was routinely performed to determine the percutaneous biopsy site. All patients provided written informed consent.
Formalin-fixed, paraffin-embedded liver tissue sections were stained by hematoxylin-eosin, Masson's trichrome, and Perls' test for iron and were evaluated by 2 pathologists (E.A. and F.A.) who were unaware of the patients' clinical and laboratory data. Liver fibrosis and degree of activity were estimated in accordance with the criteria established by Batts and Ludwing [17]. Stage of fibrosis was scored as follows: F0, no fibrosis; F1, portal fibrosis; F2, periportal fibrosis or rare portal-portal septa; F3, fibrous septa with architectural distortion and no obvious cirrhosis (bridging fibrosis); and F4, definite cirrhosis. Steatosis was graded according to the percentage of hepatocytes containing visible macrovesicular steatosis.
All data were recorded and analyzed using SPSS database software, version 11.0 (SPSS). Qualitative variables are expressed as absolute frequencies and percentages. Quantitative variables are expressed as the median and interquartile range (IQR). Numeric parameters were compared between groups using Student's t test, excepting numeric variables without normal distributions or those for small groups, in which case the numeric parameters were compared using the Mann-Whitney U test. Categorical variables were compared between groups using the Pearson x2 or Fisher's exact tests if the group was small. Differences between groups were considered to be significant when P < .05.
|
|
| |
| |
|
|
|