| |
Diagnosis/Mgt of Body Changes & Lipid Abnormalities, Hyperlactatemia
|
| |
| |
"Current Concepts in the Diagnosis and Management of Metabolic Complications of HIV Infection and Its Therapy"
Clinical Infectious Diseases Sept 1, 2006;43:645-653
D. A. Wohl,1 G. McComsey,2 P. Tebas,6 T. T. Brown,7 M. J. Glesby,9 D. Reeds,11 C. Shikuma,12 K. Mulligan,13 M. Dube,16 D. Wininger,5 J. Huang,15 M. Revuelta,10 J. Currier,14 S. Swindells,17 C. Fichtenbaum,4 M. Basar,18 M. Tungsiripat,3 W. Meyer,8 J. Weihe,12 and C. Wanke19
1University of North Carolina, Chapel Hill; 2Case Western Reserve University and 3Cleveland Clinic, Cleveland, 4University of Cincinnati, Cincinnati, and 5Ohio State University, Columbus, Ohio; 6University of Pennsylvania, Philadelphia; 7Johns Hopkins University and 8Quest Diagnostics-Baltimore, Baltimore, Maryland; 9Weill Medical College of Cornell University and 10Beth Israel Medical Center, New York, New York; 11Washington University, St. Louis, Missouri; 12University of Hawaii, Honolulu; 13University of California San Francisco, 14University of California, Los Angeles, and 15University of California, San Diego; 16Indiana University, Indianapolis; 17University of Nebraska, Omaha; and 18Frontier Science & Technology Research Foundation, Amherst, and 19Tufts University, Boston, Massachusetts
Changes in fat distribution, dyslipidemia, disordered glucose metabolism, and lactic acidosis have emerged as significant challenges to the treatment of human immunodeficiency virus (HIV) infection. Over the past decade, numerous investigations have been conducted to better define these conditions, identify risk factors associated with their development, and test potential therapeutic interventions. The lack of standardized diagnostic criteria, as well as disparate study populations and research methods, have led to conflicting data regarding the diagnosis and treatment of metabolic and body shape disorders associated with HIV infection. On the basis of a review of the medical literature published and/or data presented before April 2006, we have prepared a guide to assist the clinician in the detection and management of these complications.
CONCLUSIONS
Metabolic complications of HIV infection and its therapies are common and may compromise antiretroviral tolerability, adherence, and, ultimately, treatment success. The development of conditions that alter body shape, threaten general well-being, and require treatment with additional medications can discourage even the most motivated patient. Clinicians and patients can benefit from ongoing education regarding the risk of these complications and their relationship to antiretroviral therapies. Although there are limited data on the prevention of HIV-associated metabolic complications, it is anticipated that early detection and intervention may at least prevent progression of these conditions.
Article Text
The treatment of HIV infection with combination antiretroviral therapies has been challenged by the emergence of a number of body shape and metabolic complications. Although these complications were initially considered to be the result of antiretroviral toxicities, clinical investigations have described additional factors (such as host characteristics and direct viral effects) as being associated with their development. In addition, clinical trials have explored therapeutic approaches to these conditions, and guidelines for their therapeutic management have been published [1, 2].
On the basis of all relevant published or presented data, we have prepared this concise, evidence-based update of the current understanding of the clinical presentation, diagnosis, and management of common body shape and metabolic complications of HIV infection and its therapies (table 1). Two reviews that focus on bone disease in the setting of HIV infection were recently published, and the reader is directed to them for information regarding bone disorders [3, 4].
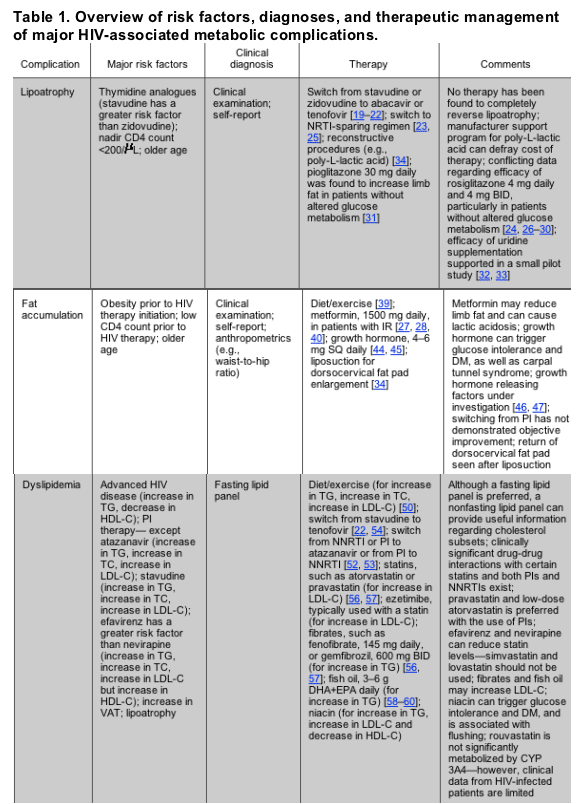
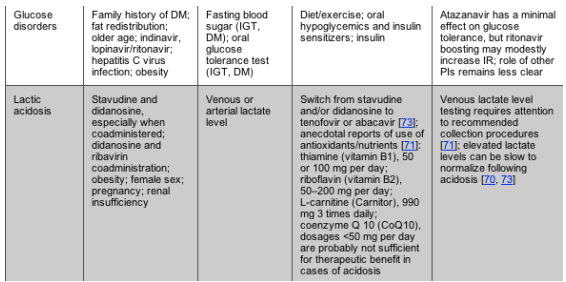
NOTE. BID, 2 times daily; CYP 3A4, cytochrome P450 3A4; DHA+EPA, docosahexaenoic acid and eicosapentaenoic acid; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; IGT, impaired glucose tolerance; IR, insulin resistance; LDL-C, low-density lipoprotein cholesterol; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; SQ, subcutaneous; TC, total cholesterol; TG, triglycerides; VAT, visceral adipose tissue.
LIPOATROPHY
Characteristics and Risk Factors
Lipoatrophy involves the loss of subcutaneous fat in the face, arms, legs, abdomen, and/or buttocks. In contrast to the traditional wasting syndrome of advancing HIV disease, lipoatrophy is distinguished by the preferential loss of fat tissue without substantial loss of lean tissue mass, and by the fact that it most frequently occurs among patients who are responding to HIV therapy [5, 6].
Antiretroviral use, disease severity, and host factors may all interact to produce lipoatrophy. Increased age and decreased CD4 cell count at initiation of HIV therapy have been associated with self-reported lipoatrophy [7]; however, objectively measured limb fat loss was associated with higher pretreatment CD4 cell counts-and not the subject's race, sex, or age-in a recent treatment trial [8]. Furthermore, age was not found to be linked to dual energy X-ray absorptiometry (DEXA)-assessed peripheral fat loss in a study of HIV-positive women undergoing HIV therapy [9]. Although some studies indicate that the risk of lipoatrophy is increased with the use of protease inhibitors (PIs) in combination with nucleoside reverse-transcriptase inhibitors (NRTIs) [10-12], therapy that consists exclusively of PIs was not found to lead to lipoatrophy [13, 14], and accumulating data demonstrate that exposure to certain NRTIs is the major factor associated with lipoatrophy [5, 15, 16] (possibly due to NRTI-induced inhibition of mitochondrial DNA polymerase y[17]). In vitro and in vivo observations indicate a differential action of NRTIs and, in particular, incriminate thymidine analogues, and stavudine in particular, in the pathogenesis of lipoatrophy [18, 19].
Screening and Diagnosis
Although a diagnosis of lipoatrophy can be made by clinical examination, patient self-report may be an early and the best indicator of body shape changes, and correlates with physical examination [6]. Anthropometric evaluations of limb circumferences and skin folds provide data regarding body shape, but these methods are limited by operator dependency. Objective quantification of fat can be obtained by CT, MRI, and DEXA scanning; however, although extremely useful for research, such imaging requires expert interpretation, is relatively expensive, and has not been observed to provide a clinical advantage over self-report and physical examination assessments. There are no widely accepted objective techniques for assessing facial lipoatrophy.
Treatment Approaches
Antiretroviral substitution. Discontinuation of PI therapy does not lead to improvement of lipoatrophy [19]. "Switch" studies with a follow-up period of 2 years have reported partial improvement in lipoatrophy after substitution of stavudine with either abacavir or tenofovir [19-22]. However, reversal of fat loss has been modest. The effect of the substitution of another NRTI for zidovudine remains inconclusive. Recent data suggest that a switch from a thymidine analogue NRTI to either abacavir or an NRTI-sparing regimen significantly increases abdominal subcutaneous fat [23]. Furthermore, increases in peripheral fat were seen in studies of lipoatrophic patients in whom a switch was made to NRTI-sparing regimens, although this occurred at the expense of a worsening lipid profile when ritonavir-boosted lopinavir was used [24].
Thiazolidinediones. Studies on the effects of these insulin-sensitizing agents on subcutaneous fat in patients with HIV infection have produced mixed results [24-31]. Two randomized, double-blind studies of rosiglitazone showed no increase in subcutaneous fat in subjects with lipoatrophy, relative to placebo [29, 30]. On the other hand, in small, randomized studies of subjects with abnormal glucose metabolism, significant increases in subcutaneous fat in subjects randomized to receive rosiglitazone have been observed, compared with placebo [24, 28] or baseline data [27]. In a preliminary report of a randomized, double-blind, placebo-controlled 48-week trial of pioglitazone in subjects required to have lipoatrophy but not insulin resistance (IR) to enter the trial, there was a modest increase in subcutaneous fat that almost achieved a level of statistical significance (P = .051); however, this effect was negated by continued stavudine use [31]. Taken together, these data suggest that there may be a role for thiazolidinediones in some patients with lipoatrophy.
Supplementation with antioxidants and mitochondrial cofactors. The use of various antioxidants and cofactors in mitochondrial metabolism have been suggested as potential therapies for lipoatrophy; however, to date, no firm conclusion has been drawn from available studies. Uridine supplementation may attenuate mtDNA decrease and may prevent apoptosis of adipose cells. In small pilot studies, the use of uridine significantly increased limb fat in patients receiving thymidine analogues, but no changes in fat and blood mitochondrial DNA were observed [32, 33].
Reconstructive surgery. Various procedures have been advocated and recently reviewed for the attenuation of facial lipoatrophy, including polylactic acid injections, fat autotransplantation, and silicone implants [34]. Polylactic-L-acid formulation (Sculptra [Sanofi-Aventis]) is approved by the US Food and Drug Administration, is often successful, and is well-tolerated with minimal adverse events, but its use has been limited by its expense.
FAT ACCUMULATION
Characteristics and Risk Factors
Accumulation of adipose tissue within the abdomen, dorsocervical fat pad ("buffalo hump"), anterior neck, and breasts have been widely reported since the advent of highly active HIV therapy (HAART) [35]. Abdominal fat accumulation is an excess of visceral adipose tissue (VAT) that results in an increase in abdominal girth. Buffalo hump results from an accumulation of fat in the dorsocervical area. Enlargement of the breasts can occur in both men and women.
A role for PIs in the development of fat accumulation is supported by the available data; however, these changes have also been observed in PI-naive patients [35, 36], and cross-sectional data have not displayed an association between increased VAT and HIV infection [37]. Longitudinal cohort studies suggest that host and disease severity factors such as increased age, higher baseline fat content, greater body mass index, white race, and low CD4 cell count at initiation of antiretroviral therapy heighten the risk for fat accumulation [35].
Screening and Diagnosis
Screening and diagnostic methods for abdominal obesity range from very simple procedures to highly sophisticated radiographic studies. In the general population, a waist/hip ratio of >0.95 in men and >0.85 in women is considered to be elevated. An elevated waist/hip ratio can be the result of either increased waist circumference or decreased hip circumference; an abdominal circumference >102 cm for men and >88 cm for women is more a precise diagnostic measurement [38]. Quantitative measurements of VAT volume can be obtained by CT or MRI but are not clinically useful. DEXA cannot distinguish between VAT and subcutaneous adipose tissue and is not a recommended screening test.
Treatment Approaches
Antiretroviral substitution. Switching from a PI to an alternative antiretroviral agent has generally not been found to reverse VAT accumulation [19].
Diet and exercise. If overall weight reduction is indicated, a reduction in energy intake (i.e., a decreased intake of saturated fat, simple sugars, and alcohol) is warranted. Fad diets and rapid weight reduction plans should be avoided. Weight reduction may be accompanied by some peripheral fat loss as well as central fat loss. Studies of HIV-positive persons demonstrate that endurance and resistance exercise can reduce VAT [39].
Metformin. In randomized studies of HIV-infected subjects with abdominal fat accumulation with [27, 40, 41] or without [42] insulin resistance, metformin therapy produced trends toward decreased VAT. However, preliminary data from another randomized study of patients with IR or glucose intolerance showed no such effect [28]. Metformin promotes a general loss of weight, such that lean body mass as well as subcutaneous fat may decrease [27, 28, 40-42].
Thiazolidinediones. There are no data to support the use of these agents for the treatment of fat accumulation.
Testosterone. Topically administered testosterone gel (10 g) in HAART-treated men with abdominal obesity and mild-moderately low testosterone levels reduced subcutaneous abdominal fat, but it also led to a loss of limb fat and had no significant effect on VAT [43].
Growth hormone. Treatment with varying doses (4-6 mg daily) of recombinant human growth hormone (rhGH) reduced total and visceral fat in trials of subjects with HIV infection [44, 45]. However, patients may develop glucose intolerance as a result of therapy. The use of rhGH is not indicated for patients with impaired glucose tolerance or with carpal tunnel syndrome. As rhGH is a potent lipolytic agent, loss of subcutaneous and peripheral fat may occur. In addition, the expense of rhGH limits access to this agent. Preliminary data suggest that the use of growth hormone-releasing factor and its analogues have favorable effects on VAT, with fewer adverse effects on glucose homeostasis than the use of rhGH [46, 47].
Reconstructive surgery. Liposuction or surgical removal of fat tissue may benefit dorsocervical fat accumulation, but regrowth of tissue is common, and bleeding, infection, and other surgical complications can occur [34].
DYSLIPIDEMIA
Characteristics and Risk Factors
Advanced HIV disease in the absence of combination antiretroviral therapy has been associated with hypertriglyceridemia, low levels of total cholesterol (TC), low levels of low-density lipoprotein cholesterol (LDL-C), and low levels of high-density lipoprotein cholesterol (HDL-C) [45]. Most PIs, with the exception of atazanavir, are associated with an elevation in levels of TC, triglycerides, and LDL-C [35]. Pharmacological boosting of atazanavir with low-dose ritonavir (100 mg) appears to increase TC and triglyceride levels beyond that of unboosted atazanavir [48]. Nonnucleoside reverse-transcriptase inhibitors (NNRTIs) produce increases in levels of TC, LDL-C, and triglycerides; however, increases in HDL-C levels may occur, particularly with nevirapine, which can yield a net reduction in the ratio of TC level to HDL-C level [49]. Among the NRTIs, stavudine is linked to increases in levels of TC, LDL-C, and triglycerides [15]. Atherogenic lipid profiles are commonly found in association with body fat changes [45].
Screening and Diagnosis
Hypertriglyceridemia and low HDL-C levels are the most common HIV-associated lipid disorders [2, 35]. Available data suggest that patients receiving HAART are at increased long-term risk of cardiovascular disease; screening for lipid disorders in HIV-infected patients is recommended [1, 2]. Fasting lipid profiles should be checked in patients prior to initiating antiretroviral therapy, and annually thereafter if significant abnormalities are not detected. When triglycerides levels are >400 mg/dL, LDL-C levels cannot be reliably calculated unless a directly measured LDL-C test is performed. In such cases, non-HDL-C levels can be calculated (the non-HDL-C level equals the TC level minus the HDL-C level) and goals for non-HDL-C levels when hypertriglyceridemia is present have been established [50].
When lipid abnormalities are present, patients should be evaluated for secondary causes of dyslipidemia (e.g., hypothyroidism, obesity, nephrotic syndrome, hypogonadism, poorly controlled diabetes mellitus [DM], heavy alcohol use, and the use of thiazide diuretics, testosterone, and oral estrogen). When elevations in triglycerides are severe, the risk for pancreatitis must be considered.
Treatment Approaches
The decision to intervene with lipid abnormalities is a complex one that must take into account the general condition and prognosis of the patient, as well as the presence of other cardiovascular disease risk factors. The National Cholesterol Education Project treatment guidelines have been revised and can be applied to persons with HIV infection [50, 51]. Additional considerations for lipid abnormality treatment in the context of HIV infection include drug-drug interactions and the option of antiretroviral manipulation, as described below.
Lifestyle modification. All patients should be counseled on lifestyle changes, including smoking cessation, adoption of a lipid-decreasing diet, and aerobic exercise, as appropriate for the individual. For patients who have not met desired lipid goals after 4-8 weeks, additional therapeutic options include change in antiretroviral regimen and initiation of lipid-decreasing therapy.
Antiretroviral substitution. Lipid levels may improve with substitution of an NNRTI for a PI in NNRTI-naive patients [19, 52]. Similarly, substitution of ritonavir-boosted atazanavir for other PIs or NNRTIs has led to improved lipid profiles [53]. Switching from stavudine to tenofovir has resulted in improvement in lipid profiles without the loss of viral suppression [54].
Lipid-decreasing therapy. When LDL-C or non-HDL-C level elevations predominate, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor (statin) therapy is preferred. However, PI treatment dramatically increases simvastatin (and presumably lovastatin) exposure, modestly increases atorvastatin exposure, and modestly decreases pravastatin exposure [55], whereas efavirenz (and probably nevirapine) treatment decreases statin exposure [56]. Pravastatin, low-dose atorvastatin, or fluvastatin are recommended as first-line agents in HIV-infected patients receiving PIs. Statin dosage may need to be cautiously increased when the statins are coadministered with efavirenz or nevirapine. The use of lipid-decreasing therapy was shown to be more effective in decreasing TC levels than switches in antiretroviral therapy [57]. When triglyceride elevations predominate and are >400 mg/dL, fibrate therapy (e.g., micronized fenofibrate or gemfibrozil) is preferred. Fish oil (3-6 g/day) was found to have significant effect on antiretroviral-associated hypertriglyceridemia but, like fibrates, may increase LDL-C levels [58-60].
Extended release formulations of niacin may decrease non-HDL-C and triglyceride levels, but may also exacerbate IR [61]. Ezetimibe, a cholesterol absorption inhibitor, decreases LDL-C levels in conjunction with statins; however, data on its use in HIV-infected patients are limited. Bile acid sequestrants are generally considered to be contraindicated because of their unknown effects on absorption of antiretrovirals and their potential to exacerbate hypertriglyceridemia.
DISORDERS OF GLUCOSE METABOLISM
Characteristics and Risk Factors
A spectrum of disorders of glucose metabolism has been associated with HIV infection and antiretroviral therapy. IR occurs when the target tissues of insulin action fail to respond appropriately to insulin, resulting in increased pancreatic insulin production. Impaired glucose tolerance is an elevated blood sugar level of 140-199 mg/dL 2 h after receipt of a 75-g loading dose of glucose during an oral glucose tolerance test [62]. Impaired fasting glucose tolerance occurs when the fasting blood sugar level is in the 100-125-mg/dL range. The presence of impaired glucose tolerance or impaired fasting glucose tolerance suggests that IR may be present. DM is diagnosed when the fasting blood sugar level is 126 mg/dL, or the 2-h oral glucose tolerance test glucose level is >200 mg/dL and is confirmed by additional testing, or when a patient has symptoms of DM (frequent urination, thirst, blurred vision, or weight loss) in the setting of a blood glucose level >200 mg/dL.
Risk factors for the development of disorders of glucose metabolism include obesity, lipoatrophy, use of most PIs, NRTI exposure (particularly stavudine), older age, family history of DM, nonwhite race, and possibly hepatitis C virus coinfection [63, 64]. Other data suggest that traditional risk factors for IR are more important than treatment-related factors [65]. Use of niacin, growth hormone, corticosteroids, and antipsychotics may also contribute to hyperglycemia.
Screening and Diagnosis
A fasting (>8 h) blood glucose level should be checked before initiation of HIV therapy and should be monitored every 3-6 months for patients with changes in treatment regimen or who have significant risk factors for IR. For patients with impaired glucose tolerance or who have risk factors for DM, a 2-h oral glucose tolerance test should be considered. There are no recommended laboratory tests for the diagnosis of IR, and the variability among different insulin assays has made an establishment of a defined cutoff level difficult. Therefore, insulin levels should generally not be relied upon to diagnose IR. Dyslipidemia and body fat abnormalities are often accompanied by IR, and for patients with these symptoms, a 2-h oral glucose tolerance test should be considered.
Treatment Approaches
Lifestyle changes. Dietary guidelines established for the HIV-uninfected patient are relevant for the management of glucose disorders in the context of HIV infection [62]. Weight loss through increased activity and caloric restriction should be recommended for overweight HIV-infected patients with abnormalities in glucose metabolism.
Pharmacology. In general, the management of glucose disorders in patients with HIV infection does not differ from that of HIV-uninfected patients; thus, relevant guidelines should be applied [62]. As in the general population, medications should be reserved for patients who have established DM. Metformin improves insulin sensitivity in patients with HIV lipodystrophy [27, 28, 40, 41] and is an effective antidiabetic medication. Because development of lactic acidosis is a rare but serious side effect, this drug should be used with caution in patients receiving an NRTI, and it is contraindicated for persons with impaired renal function. Thiazolidinediones improve insulin sensitivity in patients with HIV lipoatrophy and are a reasonable choice for the treatment of DM in the context of HIV infection [24, 27, 28]. Weight gain and fluid retention is common with these agents, and rosiglitazone treatment may worsen hyperlipidemia [24, 29, 30]. Sulfonylureas improve plasma glucose by stimulating insulin secretion but do not reverse underlying IR. Insulin is inexpensive, well tolerated, and effective for the treatment of DM, particularly when the response to oral agents has been suboptimal. Regardless of therapeutic approach, the goal of therapy should be the normalization of glycosylated hemoglobin. In many cases, achievement of glycemic control may require combination therapy.
Antiretroviral substitution. Substitution of an NNRTI for a PI has been observed to increase insulin sensitivity in some-but not all-studies [19].
LACTIC ACIDOSIS/SYMPTOMATIC HYPERLACTATEMIA
Characteristics and risk factors. Lactic acidosis is an uncommon but potentially fatal complication of treatment with NRTIs. A milder syndrome with a substantially better prognosis, characterized by symptomatic hyperlactatemia without acidosis, has also been reported [66]; however, other data suggest that most cases of asymptomatic hyperlactatemia are the artifacts of suboptimal collection of venous lactate [67]. The putative pathophysiological mechanism of this syndrome involves NRTI-mediated mitochondrial dysfunction due to the inhibition of mitochondrial DNA polymerase with excess production of lactate as a consequence of anaerobic glycolysis [17]. Although lactic acidosis has been reported with the use of all NRTIs, an increased risk is associated with the use of either stavudine or didanosine, and the risk appears to be heightened when these are coadministered [68-70].
Symptoms may be acute to subacute and include fatigue, weight loss, abdominal pain, nausea or vomiting, and compensatory hyperventilation. Mild to moderate elevations of liver enzyme levels are common and may be associated with hepatic steatosis. Concomitant pancreatitis may occur. Reported risk factors for lactic acidosis/symptomatic hyperlactatemia, in addition to stavudine and didanosine treatment, include female sex, obesity, and the use of ribavirin with didanosine in patients with hepatitis C virus coinfection [70].
Diagnosis and screening. Lactate levels can be measured in venous blood specimens if collection procedures are rigorously followed [71]; these include collection of the specimen without use of a tourniquet and not permitting the patient to clench their fist prior to phlebotomy. Persistent hyperlactatemia is rare in asymptomatic HIV-infected patients and is not predictive for future symptomatic lactic acidosis [67, 72]. It has been hypothesized that use of other mitochondrial toxic agents, alcohol, or intercurrent illness (such as acute respiratory disease) may act as triggers to the development of symptomatic disease in patients with previously normal or stable lactate levels.
Treatment approaches. A high index of suspicion, prompt recognition, and discontinuation of offending NRTIs are the hallmark of successful therapy for lactic acidosis/symptomatic hyperlactatemia. Routine assessment of plasma lactate levels in asymptomatic HIV-infected patients is not warranted. Substitution of the offending antiretroviral regimen with NRTI-sparing regimens or, alternatively, regimens involving NRTIs with less mitochondrial toxicity potential are appropriate [73]. Although case studies have reported patient improvement with the use of adjunctive therapies (e.g., antioxidants, vitamin B supplements, and dichloroacetate) during the acute phase of the syndrome, no controlled studies exist to substantiate their efficacy.
|
|
| |
| |
|
|
|