| |
Liver Related Disease Causes Most Frequent Non-AIDS Deaths; 76% Due to HCV or HBV Coinfection
|
| |
| |
Arch Intern Med. Aug 14/28, 2006
".....In this large prospective study (D.A.D), we found that AIDS-related disease still accounted for most deaths (31.1%), and liver disease was the most frequent non-AIDS-related cause (14.5%)..... More than 76% of the liver-related deaths in the present study were associated with infection with either HBV or HCV.... There was a strong association between immunodeficiency (lower CD4 counts) and an elevated risk of liver-related and other non-AIDS-related deaths...... there may be negative effects of cART on the liver in addition to the positive effects acting via CD4 cell count increases.......Antiretroviral therapy has been reported to reduce liver-related mortality in HCV-coinfected persons,32 but we found no such effect.... In the present cohort, 2.7% of all liver-related deaths were reported to be directly associated with antiretroviral medication. The link with acute hepatotoxicities for some antiretroviral drugs is an undisputed but infrequent cause of mortality...... more than half of those who died of liver disease had undetectable viral loads at the time of death....
...In a prospective cohort study of 23 441 HIV-infected persons, the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group investigated 181 liver-related deaths, which were the most frequent cause of non-AIDS-related deaths. There was a strong association between immunodeficiency and an elevated risk of liver-related and other non-AIDS-related deaths. Other strong associations with liver-related deaths were intravenous drug use, HCV infection, and active HBV infection. Many patients in the D:A:D study who died had initiated monotherapy or dual therapy at a time when cART was not available. Only a few of those who died had never received cART....
....A total of 1246 persons (5.3%) died (incidence, 1.62 per 100 person-years; 95% confidence interval [CI], 1.53-1.71 per 100 person-years) at a median age of 44 years (IQR, 38-52 years); 5.2% of those who died had never received ART. The most common causes of death were AIDS (31.1% of deaths; incidence, 0.51 per 100 person-years), liver-related diseases (14.5% of deaths; incidence, 0.24 per 100 person-years), cardiovascular or other heart diseases (11.0% of deaths; incidence, 0.18 per 100 person-years), and non-AIDS malignancies (9.4% of deaths; incidence, 0.15 per 100 person-years); 33.8% of deaths were from other causes (incidence, 0.55 per 100 person-years)....
....When the CD4 cell count and HIV RNA level were included in the model as continuous variables, a 2-fold lower CD4 cell count was associated with a 23% increased risk of liver-related death (adjusted relative hazard per 2-fold lower CD4 cell count, 1.23; 95% CI, 1.17-1.29; P<.001), and each log higher HIV RNA level was associated with an increased risk of 27% (relative hazard, 1.27; 95% CI, 1.13-1.43; P<.001). The effects of other factors in the model were unchanged after including the variables in this way....
...There was a strong relationship between immunodeficiency and liver-related death after adjusting for other potential confounding variables (adjusted relative rates [RRs] of 16.06, 11.54, 7.14, 3.95, and 1.67 for those with latest CD4 cell counts of <50, 50-99, 100-199, 200-349, and 350-499/μL, respectively, compared with CD4 cell counts 500/μL) (Figure 1). Other independent predictors of liver-related death were older age (adjusted RR per 5 years, 1.32), HIV acquisition via intravenous drug use (adjusted RR, 2.01), HCV infection (adjusted RR, 6.66), and active HBV infection (adjusted RR, 3.73)....
"Liver-Related Deaths in Persons Infected With the Human Immunodeficiency Virus"
The D:A:D Study
The Data Collection on Adverse Events of Anti-HIV Drugs Study Group*
Arch Intern Med. 2006;166:1632-1641.
Vol. 166 No. 15, Aug 14/28, 2006
More than 50% of all deaths in HIV-infected persons receiving cART are now from causes other than AIDS.2, 8-10 An increasing proportion of deaths are the result of chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) infections and intravenous drug and alcohol use. In addition, there is an increasing awareness of the potential toxic effects associated with prolonged cART. Although some adverse effects (eg, lactic acidosis) are now rarely observed owing to avoidance of specific drug combinations,11-12 long-term toxic effects possibly resulting from mitochondrial toxicity, type 2 diabetes mellitus, and cardiovascular disease have emerged.12-14 The effect of any cART-induced hepatotoxicity15-18 on the risk of liver-related mortality remains to be determined. We investigated the causes and frequency of death in a large multicenter cohort of HIV-infected individuals and assessed the relationships between liver-related deaths and HIV-associated immunodeficiency, HBV and HCV coinfections, and cART.
Background An increasing proportion of deaths among human immunodeficiency virus (HIV)-infected persons with access to combination antiretroviral therapy (cART) are due to complications of liver diseases.
Methods We investigated the frequency of and risk factors associated with liver-related deaths in the Data Collection on Adverse Events of Anti-HIV Drugs study, which prospectively evaluated 76 893 person-years of follow-up in 23 441 HIV-infected persons. Multivariable Poisson regression analyses identified factors associated with liver-related, AIDS-related, and other causes of death.
Results
There were 1246 deaths (5.3%; 1.6 per 100 person-years); 14.5% were from liver-related causes.
Of these, 16.9% had active hepatitis B virus (HBV), 66.1% had hepatitis C virus (HCV), and 7.1% had dual viral hepatitis co-infections.
Predictors of liver-related deaths were latest CD4 cell count (adjusted relative rate [RR], 16.1; 95% confidence interval [CI], 8.1-31.7 for <50 vs 500/μL), age (RR, 1.3; 95% CI, 1.2-1.4 per 5 years older), intravenous drug use (RR, 2.0; 95% CI, 1.2-3.4), HCV infection (RR, 6.7; 95% CI, 4.0-11.2), and active HBV infection (RR, 3.7; 95% CI, 2.4-5.9).
Univariable analyses showed no relationship between cumulative years patients were receiving cART and liver-related death (RR, 1.00; 95% CI, 0.93-1.07).
Adjustment for the most recent CD4 cell count and patient characteristics resulted in an increased risk of liver-related mortality per year of mono or dual antiretroviral therapy before cART (RR, 1.09; 95% CI, 1.02-1.16; P = .008) and per year of cART (RR, 1.11; 95% CI, 1.02-1.21; P = .02).
Conclusions
Liver-related death was the most frequent cause of non-AIDS-related death. We found a strong association between immunodeficiency and risk of liver-related death. Longer follow-up is required to investigate whether clinically significant treatment-associated liver-related mortality will develop.
COMMENT
In this large prospective study, we found that AIDS-related disease still accounted for most deaths (31.1%), and liver disease was the most frequent non-AIDS-related cause (14.5%). There was a strong association between immunodeficiency and an elevated risk of liver-related and other non-AIDS-related deaths. Other strong associations with liver-related deaths were intravenous drug use, HCV infection, and active HBV infection. Many patients in the D:A:D study who died had initiated monotherapy or dual therapy at a time when cART was not available. Only a few of those who died had never received cART.
Liver-related deaths are largely a consequence of chronic HBV or HCV coinfection,9-10,20-24 although the association between HCV coinfection and increased mortality has not consistently been identified.25-26 More than 76% of the liver-related deaths in the present study were associated with infection with either HBV or HCV. Infection with HIV accelerates the progression of HCV disease to cirrhosis presumably by increasing hepatitis C viremia.27-28 Conversely, HCV may adversely affect HIV infection and reduce the effectiveness of cART,29 although this remains controversial.30-31 Whereas HBV infection increases liver-related mortality in coinfected persons, it does not seem to affect the progression of HIV or the response to cART.23 Antiretroviral therapy has been reported to reduce liver-related mortality in HCV-coinfected persons,32 but we found no such effect.
In the present cohort, 2.7% of all liver-related deaths were reported to be directly associated with antiretroviral medication. The link with acute hepatotoxicities for some antiretroviral drugs is an undisputed but infrequent cause of mortality.15-16 In contrast, the possible long-term adverse effects of cART on hepatic function are controversial,15, 17 although a recent study33 found a 12% increase in liver-related deaths per year of cART. Medication-associated hepatotoxicity may be more frequent in HCV-coinfected persons.15, 34 The present results do not allow us to reach firm conclusions about any relationship between prolonged cART and liver-related death. Because a higher CD4 cell count is associated with a lower rate of liver death, cART might affect the liver death rate in at least 2 ways: (1) by increasing the CD4 cell count, and thus reducing the death rate and (2) by increasing the death rate due to putative long-term hepatotoxicity. This analysis, which assessed the relationship between cumulative cART exposure and the liver death rate without accounting for follow-up CD4 cell counts, suggests no association. Thus, if either of the 2 possible effects is operating, then it may be negated by the other. In contrast, adjusting for the latest CD4 cell count, which can be interpreted as showing the remaining relationship between cART duration and liver-related mortality after the positive effects of therapy have been removed, showed an 11% (95% CI, 2%-21%; P = .02) increased rate of liver-related death per year of cART. Thus, there may be negative effects of cART on the liver in addition to the positive effects acting via CD4 cell count increases. However, the residual relationship was relatively modest and of borderline statistical significance, so it is difficult to rule out confounding. The use of mono or dual ART before cART was also an independent factor seen among liver-related deaths, but adjustment for this variable did not modify the relationship between cART and liver-related deaths. This finding, however, might suggest that the prolonged use of reverse transcriptase inhibitors is a risk factor for hepatotoxicity. Further follow-up and increasing numbers of liver-related deaths are needed before the mechanisms behind our results can be clarified.
Although it has been recognized that HIV infection considerably accelerates the progression of HCV disease,27 we were surprised to find such a strong relationship between liver-related deaths and cellular immunodeficiency. Although HIV replication was also predictive of these deaths, more than half of those who died of liver disease had undetectable viral loads at the time of death. A similar relationship between immunosuppression and poorer outcome is also seen in HIV-uninfected, HCV-infected liver transplant recipients: more intense posttransplantation immunosuppression, following the use of corticosteroids and antiproliferative agents, leads to increased graft failure and progression to cirrhosis.35 In these patients, treatment for rejection has been associated with diminished survival in HCV-infected but not HCV-seronegative recipients.36
The present study has notable strengths, in particular its large size, broad geographic area, inclusion of a representative group of persons with HIV infection, and rigorous review and central coding of causes of death. Nonetheless, some specific causes of death are still too infrequent to permit an analysis of risk factors. In line with all clinical studies, only a small proportion of patients underwent autopsy after death, and many patients died outside a medical institution, hampering assessment of causes of death. Moreover, information on alcohol intake was not routinely available and could not be considered, although it is an important risk for liver disease.37
There are a variety of clinical implications of these findings. First, the strong association between immunodeficiency and an elevated risk of liver-related death underlines the importance of HIV treatment strategies that prevent immunodeficiency. These findings suggest that the effect of immunodeficiency on the risk of these types of death remains present even in patients with CD4 cell counts of 200 to 500/μL. Future studies should explore the possible benefits and risks of starting ART at CD4 cell counts higher than currently recommended in patients with a known risk of liver-related death. Second, the question of if and when to treat hepatitis co-infections must consider the status of liver disease and the immune status. Third, treatment or prevention of illicit drug and alcohol use is particularly important in HCV-coinfected persons because these risks are associated with accelerated progression to liver cirrhosis.
In conclusion, although the pattern of death in HIV-infected persons has changed, mortality rates remain substantial. Liver diseases, mainly due to hepatitis virus infections, accounted for almost 15% of the deaths and were strongly associated with advanced immunodeficiency. There was no definite relationship between mortality and ART. The possibility of increased medication-related mortality cannot be excluded, but the CD4 cell count increases with cART seem to balance any adverse effects that exist. Combination ART has substantially improved the outcomes of HIV-infected persons with access to care.1, 3-5 However, long-term studies of large cohorts are crucial for the systematic collection and analyses of clinical end points to investigate the relationships between immune function and end-organ failure8, 38 and to recognize novel and late-emerging treatment-related toxic effects.
RESULTS
PATIENT CHARACTERISTICS
We followed up 23 441 persons for a median of 3.5 years (interquartile range [IQR], 3.1-3.8 years; 76 893 person-years of follow-up). Follow-up on 20.8% of the participants was censored 6 months after their last cohort visit. At enrollment in the D:A:D study, participants had a median age of 39 years (range, 34-45 years), and 24.1% were women. The median nadir CD4 cell count before enrollment was 200/μL (range, 1-2580/μL), and clinical AIDS had been diagnosed in 26.4% of the participants. A total of 22.5% of the patients were HCV positive, and 6.8% had active and 21.4% had inactive HBV infection. Hepatitis C virus positivity and active HBV infection were found in 1.6% of the patients. By February 1, 2004, 88.7% of the patients had received cART (median, 4.5 years; range, 2.3-6.3 years), of whom 48.1% had received monotherapy or dual combination therapy before initiating cART; 4.4% of the patients had received therapy with nucleoside reverse transcriptase inhibitors only, either as monotherapy or dual therapy or as part of triple nucleoside reverse transcriptase inhibitor combinations.
MORTALITY AND LATEST CD4 CELL COUNTS
A total of 1246 persons (5.3%) died (incidence, 1.62 per 100 person-years; 95% confidence interval [CI], 1.53-1.71 per 100 person-years) at a median age of 44 years (IQR, 38-52 years); 5.2% of those who died had never received ART. The most common causes of death were AIDS (31.1% of deaths; incidence, 0.51 per 100 person-years), liver-related diseases (14.5% of deaths; incidence, 0.24 per 100 person-years), cardiovascular or other heart diseases (11.0% of deaths; incidence, 0.18 per 100 person-years), and non-AIDS malignancies (9.4% of deaths; incidence, 0.15 per 100 person-years); 33.8% of deaths were from other causes (incidence, 0.55 per 100 person-years).
In the 1246 patients who died, the latest CD4 cell count before death was less than 50/μL in 24.5% and less than 200/μL in 54.2%. The latest CD4 cell count was measured a median of 10.7 weeks (IQR, 5.3-18.4 weeks) before death (in those with a latest CD4 cell count <200/μL, the assessment was a median of 10.1 weeks [IQR, 5.3-18.3 weeks] before death, and in those with a latest CD4 cell count of >200/μL, the assessment was 11.1 weeks [IQR, 5.1-18.6 weeks] before death). We found a strong relationship between the degree of cellular immunodeficiency and AIDS-related, liver-related, and all other deaths (Table 1).
CLINICAL PRESENTATION
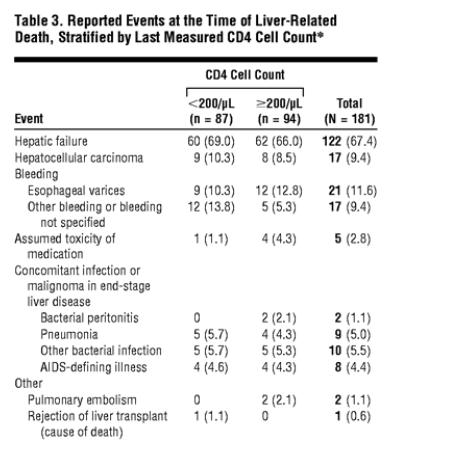
We observed 181 liver-related deaths: 87 (48.1%) in patients with CD4 cell counts less than 200/μL and 94 (51.9%) in those with CD4 cell counts of 200/μL or greater. Autopsy results were available for 9.8% of these patients. The characteristics of persons who died are given in Table 2. Individuals who died of liver-related causes were more likely to have acquired HIV infection by intravenous drug use and were more likely to have documented HCV coinfection or active HBV coinfection or HCV and active HBV coinfection. The median nadir and latest CD4 cell counts of patients dying of liver-related causes were higher than those of patients dying of AIDS. Although a smaller proportion of patients had started cART with a low CD4 cell count, a greater proportion had received monotherapy or dual therapy before cART. They were equally likely to be receiving treatment with an undetectable HIV RNA level at the time of death. Reported diagnoses at the time of death are summarized in Table 3. There were no differences in the characteristics of liver-related deaths between patients with CD4 cell counts less than 200/μL vs 200/μL or greater.
RISK FACTORS
There was a strong relationship between immunodeficiency and liver-related death after adjusting for other potential confounding variables (adjusted relative rates [RRs] of 16.06, 11.54, 7.14, 3.95, and 1.67 for those with latest CD4 cell counts of <50, 50-99, 100-199, 200-349, and 350-499/μL, respectively, compared with CD4 cell counts >500/μL) (Figure 1). Other independent predictors of liver-related death were older age (adjusted RR per 5 years, 1.32), HIV acquisition via intravenous drug use (adjusted RR, 2.01), HCV infection (adjusted RR, 6.66), and active HBV infection (adjusted RR, 3.73).
When the CD4 cell count and HIV RNA level were included in the model as continuous variables, a 2-fold lower CD4 cell count was associated with a 23% increased risk of liver-related death (adjusted relative hazard per 2-fold lower CD4 cell count, 1.23; 95% CI, 1.17-1.29; P<.001), and each log higher HIV RNA level was associated with an increased risk of 27% (relative hazard, 1.27; 95% CI, 1.13-1.43; P<.001). The effects of other factors in the model were unchanged after including the variables in this way.
A total of 578 patients (2.5%) had started HCV treatment, and 20 735 patients (88.5%) had used 1 of the 3 ART drugs (lamivudine, tenofovir disoproxil, or adefovir dipivoxil) also active against HBV infection. Sensitivity analyses adjusting for specific treatment of HCV or HBV infection did not substantially change the factors observed in the main model.
LIVER-RELATED DEATHS AND DURATION OF cART
Liver-related death rates according to duration of cART and stratified by the latest CD4 cell counts and HCV status are shown in Figure 2. Death rates remained stable during the first 7 years of cART. Univariable analyses confirmed that there was no relationship between cumulative cART and liver-related deaths either overall (RR, 1.00; 95% CI, 0.93-1.07 per year of exposure; P = .93) or in CD4 cell count (<200/μL: RR, 1.08; 95% CI, 0.96-1.20; P = .20; >200/μL: RR, 0.98; 95% CI, 0.88-1.08; P = .66) or HCV (negative: RR, 0.99; 95% CI, 0.87-1.12; P = .85; positive: RR, 1.01; 95% CI, 0.92-1.10; P = .82) strata.
Adjustment for time-updated antiretroviral monotherapy or dual therapy initiated before cART, the nadir CD4 cell count (as a continuous log2-transformed variable), and other clinical and demographic variables (Figure 1) modified the relationship with cART slightly (Table 4). Adjustment for the latest CD4 cell count, which allows for study of the effect of cumulative cART over and above any positive effects of cumulative cART on CD4 cell counts, resulted in an increased risk of liver-related mortality with longer exposure to cART (1.11; P = .02) The use of mono or dual ART before cART was associated with an increased risk of liver-related deaths, but adjustment for this variable did not modify the relationship between cART and liver-related deaths.
METHODS
DESIGN
The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study is a collaborative, observational study of 11 cohorts that includes 23 441 HIV-1-infected persons prospectively followed up at 188 clinics in Europe, the United States, and Australia.19 The study began in December 1999, and the latest follow-up for this analysis is February 2004. Although the primary end point of the study is myocardial infarction, other end points, including all deaths, are prospectively collected. Details of quality assurance, governance, and sponsorship have been published elsewhere.14
DATA COLLECTION
All the participants were under prospective follow-up in their individual cohorts at the time of enrollment in the D:A:D study. Standardized data collection forms containing sociodemographic, clinical, laboratory, and comprehensive antiretroviral and other medication information are completed at each site annually. Results of HBV and HCV serologic and viral load assessments, if available, have been collected since January 1, 2004 (including previously collected data by the participating cohorts). Information on cause of death was collected using a case report form and was reviewed and coded centrally at the study coordinating office.
END POINTS
Deaths were classified as "liver related" if clinical or autopsy diagnoses described hepatic failure (regardless of etiology), hepatocellular carcinoma in patients with end-stage liver disease, esophageal or other bleeding in the presence of end-stage liver disease, assumed medication-related hepatotoxicity resulting in hepatic failure without evidence of other etiologies of hepatic failure, or acute hepatic failure after acute bacterial or opportunistic infection in patients with previously known end-stage liver disease. Deaths due to opportunistic infections or malignancies according to the Centers for Disease Control and Prevention definition of clinical AIDS were classified as "AIDS related." The category "non-AIDS malignancies" excluded AIDS-defining and hepatitis virus-associated malignancies.
STATISTICAL ANALYSIS
All the analyses were based on calculations of the incidence rate, defined as the number of deaths divided by the total person-years of follow-up. Follow-up began at enrollment in the D:A:D study and ceased on the date of death, February 1, 2004, or 6 months after the patient's last cohort visit, whichever came first. Each person's follow-up was divided into a series of consecutive 1-month periods, and his or her status regarding each explanatory variable was determined at the start of each month. Explanatory variables considered were sex, cohort, HIV transmission group, ethnicity (all fixed covariates that remained unchanged), age, body mass index, family history of cardiovascular disease, smoking status, previous cardiovascular event, HCV status (negative: seronegative, or seropositive but HCV RNA negative; positive: seropositive and HCV RNA positive or HCV RNA unknown; or not tested), HBV status (positive: active infection [HB surface antigen, HB e antigen, or HBV DNA positive]; positive: inactive infection [HB surface antigen negative, anti-HB e antibody positive, or HBV DNA negative]; vaccinated: anti-HB surface antigen positive only; or not tested), latest available CD4 cell count, latest available HIV RNA level, and calendar year (all time updated so that the value used was that from the beginning of the month in question rather than that from the time of D:A:D enrollment). Poisson regression models were used to quantify the relationship between each factor and the rate of death from each cause.
Each cause of death was modeled separately in all the analyses, and, therefore, the characteristics of patients dying of each cause were compared with those of all other patients in the cohort (including those dying of other causes). To rule out the possible competing effects of death from another cause, all the analyses were repeated after excluding from each those who died of other causes (eg, for analyses of the factors associated with liver-related deaths, individuals dying of AIDS-related and other causes were excluded). The conclusions remained unchanged.
To investigate the relationship between increased exposure to cART and liver-related deaths, we initially fitted a univariable Poisson regression model with cART categorized as 0, less than 1, 1 to less than 2, 2 to less than 3, 3 to less than 4, 4 to less than 5, 5 to less than 6, and 6 or more years, and then years of cART was considered as a continuous covariate. Subsequent analyses controlled for time-updated exposure to mono or dual antiretroviral therapy (ART) initiated before cART, the nadir CD4 cell count (and all other variables described previously), and then the latest CD4 cell count. The nadir CD4 cell count, the latest CD4 cell count (both after log2 transformation), and the latest HIV RNA level (after log10 transformation) were included as continuous covariates in these analyses. Additional analyses considered cumulative exposure to cART and liver-related mortality after stratifying by the latest CD4 cell count and the patients' HCV status and after lagging the CD4 cell count by 3 or 6 months (ie, taking the CD4 cell count value for any 1 month as the value held 3 [or 6] months ago rather than the latest value).
|
|
| |
| |
|
|
|