| |
Lack of Decay of HIV-1 in (GALT) Gut-Associated Lymphoid Tissue Reservoirs in Maximally Suppressed Individuals
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 43(1) September 2006 pp 65-68
Poles, Michael A. MD, PhD ; Boscardin, W. John PhD*; Elliott, Julie MS*; Taing, Philip BS*; Fuerst, Marie M.P. MS, RN*; McGowan, Ian MD, PhD*; Brown, Stephen MD ; Anton, Peter A. MD*
From the *Center for Prevention Research at David Geffen School of Medicine, University of California-Los Angeles AIDS Institute, University of California-Los Angeles, Los Angeles, CA; Aaron Diamond AIDS Research Center, New York University School of Medicine, New York, NY; and AIDS Research Alliance, West Hollywood, CA.
AUTHOR DISCUSSION
We undertook this study to determine whether detectable levels of HIV-1 DNA or RNA remain in the GI mucosa of HIV-1-infected, well-suppressed subjects on HAART with long durations of undetectable plasma HIV-1 and whether we could identify decay in those levels. We were also able to use data from these subjects to calculate the potentially replicative viral burden of HIV-1 in GALT in these subjects, as a reflection of a sustainable reservoir. Study subjects had received HAART for an average of 4 years; although HIV-1 RNA was detected in mucosal biopsy specimens from only 20% of the subjects, mucosal HIV-1 DNA was detected in 80% of the subjects. There was no evidence for decay of the HIV-1 DNA in either GALT or PBMCs.
Tissue reservoirs of latently infected CD4 cells have been previously characterized in peripheral blood and lymph nodes.14 Gastrointestinal mucosal cells that contain HIV-1 DNA may represent a significant additional viral reservoir. Because of the compartment's physiologic state of activation and negligible frequency of resting T cells, true latency is not likely present, although chronic, low-level persistent replication is. In our study, we calculated the presence of 3.5 replication-competent HIV-1 DNA-positive cells per million PBMCs, a value similar to that from others.15 Based on simultaneously obtained samples of gut mucosa, we calculated a value of 0.36 replication-competent HIV-1 DNA-positive cells per million gut cells. Using conservative estimates and assumptions, the total number of concurrent HIV-1 DNA- positive cells was calculated to be 70,000 in PBMCs and 160,000 in GALT. This indicates that GI mucosa is a quantitatively important secondary compartment of potentially replication-competent cells containing HIV-1 DNA. We did not attempt to induce viral replication in the samples from this study; mucosal coculture is extremely difficult, and subset cellular yield for coculture is low from biopsy specimens. We have, however, previously shown that replication-competent HIV-1 virions can be produced from such samples.4
The lack of any statistically significant decay of HIV-1 DNA or RNA in the PBMCs and mucosal compartment in our subjects is consistent with previous reports dealing with PBMCs and lymph nodes.16 Our findings indicate that-despite prolonged treatment with HAART-the detected long half-life estimates of HIV-1 DNA in GALT reflect those seen in PBMCs and lymph nodes and further support "cryptic replication" and the low likelihood of eradication efforts with currently available therapies.
Abstract
Summary: Although peripheral blood mononuclear cells (PBMCs) and lymph nodes represent a principal reservoir, the contribution of gut-associated lymphoid tissue (GALT) has not been evaluated. In 15 HIV-1-infected subjects with maximal suppression of HIV replication by highly active antiretroviral therapy, we quantified HIV-1 DNA and RNA in mucosal biopsy specimens, PBMCs, and plasma with ultrasensitive assays.
We also calculated compartmental burdens of HIV-1 DNA-positive cells and characterized the temporal decay of these reservoirs in a period of 1 year (with projections to >50 years).
HIV-1 RNA was detected in 20% of the subjects' mucosal biopsy specimens and in 80% of the PBMC samples.
Mucosal HIV-1 DNA was detected in 80% of the subjects and in 100% of the PBMC samples.
Calculated numbers of lymphoid cells containing "potentially replication-competent" HIV-1 DNA showed that the PBMC compartment contained approximately 70,000 such cells, and GALT contained approximately 160,000 cells.
Rates of decay slopes for all 15 subjects in both compartments were not statistically significantly different when compared with each other or with zero slope.
Our data indicate that GALT is a quantitatively important reservoir of potentially replicative cells containing HIV-1 DNA, harboring at least as many or more of such cells as the PBMC compartment. In well-suppressed patients on highly active antiretroviral therapy, the GALT compartment showed no clear pattern of HIV-1 decay, similar to that in the PBMCs.
HIV-1 latency has been defined as a reversibly nonproductive infection-usually of CD4+ resting memory T cells. Chronic persistent HIV-1 can also be maintained by intermittent activation or "cryptic" low-level viral replication.1,2 Viral eradication has not been achieved because of the presence of true latently infected cells or those with ongoing but cryptic replication, even when effective therapy suppresses HIV-1 RNA in blood to undetectable levels. Lymphoid tissue compartments have been shown to be the main site of residence of latently infected CD4 cells with replication-competent but transcriptionally silent viral genomes.
The role of the gastrointestinal (GI) mucosa has not been quantified as an additional reservoir of HIV-1-infected cells. This compartment contains both a primary immune component of disaggregated, activated memory cells and more organized secondary immune tissue known as GALT (gut-associated lymphoid tissue). Because GALT is the largest immune organ in the body and most GALT CD4 cells are in an activated (not resting), memory state,3 we evaluated the contribution of GALT as a reservoir pool of HIV-1-infected cells in well-suppressed subjects on highly active antiretroviral therapy (HAART). We also performed longitudinal analyses to test for decay of viral load in plasma, peripheral blood mononuclear cells (PBMCs), and GALT. Our findings indicate that in 15 subjects who received HAART and who had undetectable levels of plasma HIV-1 RNA, GALT is a major reservoir of HIV-1-infected cells. No appreciable decay of HIV-1 DNA-containing cells in this or the PBMC compartment could be detected during a 12-month observation period.
RESULTS
Subject Characteristics at Baseline
Subjects were scheduled for return visits at 3, 6, 9, and 12 months. As a composite, the 15 subjects were seen in a total of 59 (79%) of the possible 75 visits, usually with sampling from all 3 compartments (colonic mucosa, PBMCs, and plasma). Their mean ± SD age was 45.5 ± 6.8 years. Their mean ± SD CD4 cell count was 581.9 ± 327.0 cells/mm3; subjects had been diagnosed HIV-1 seropositive for a mean ±SD of 131.2 ± 42.2 months before study enrollment. The mean ± SD duration of HAART was 46.3 ± 23.1 months. None of the subjects underwent any change in therapy during the study.
Mucosal and PBMC HIV-1 DNA and RNA
HIV-1 DNA was detected in all PBMC samples. In the colonic mucosa, HIV-1 DNA was detected in 41 (70%) of 59 samples, representing 12 (80%) of the 15 subjects. HIV-1 RNA was detected in 33 (79%) of 42 PBMC samples from 12 of the 15 subjects. Only 9 (15%) of 59 mucosal samples yielded detectable HIV-1 RNA, which represented 3 subjects (20%) (Fig. 1).
Mucosal and PBMC HIV-1 DNA and RNA
HIV-1 DNA was detected in all PBMC samples. In the colonic mucosa, HIV-1 DNA was detected in 41 (70%) of 59 samples, representing 12 (80%) of the 15 subjects. HIV-1 RNA was detected in 33 (79%) of 42 PBMC samples from 12 of the 15 subjects. Only 9 (15%) of 59 mucosal samples yielded detectable HIV-1 RNA, which represented 3 subjects (20%) (Fig. 1).
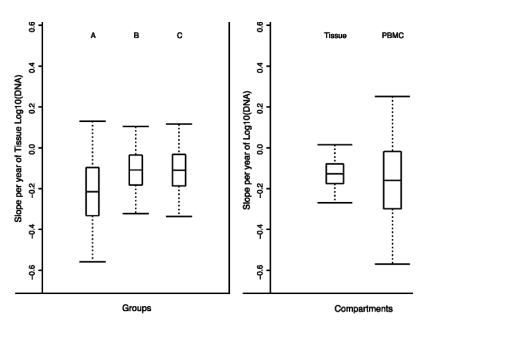
FIGURE 1. Summary of slopes of changing levels of HIV DNA in study groups and compartments. A, Estimated mean slopes (horizontal line in box plot) and 95% confidence intervals (whiskers) for the more than 12-month study periods for tissue HIV DNA in groups A, B, and C. The box bounds a 50% confidence interval. The estimated slope is slightly negative in each of the 3 groups (mean is <0) but not statistically distinguishable from zero. B, Estimated mean slopes and 95% confidence intervals for tissue HIV DNA and PBMC HIV DNA in all study subjects. The estimated mean slope is slightly negative but not statistically distinguishable from zero in both compartments. The relative sparsity of PBMC studies (n = 26) versus tissue studies (n = 59) is reflected in the larger width of the confidence interval for the slope of PBMC HIV DNA.
Clinical Correlates of HIV-1 Detection
All clinical parameters assessed (CD4 cell count, months of HIV-1 infection, and duration of HAART) showed only weak or moderate correlations with measurements of viral burden (as determined by HIV-1 DNA in PBMCs or mucosa and HIV-1 RNA in PBMCs). None of these correlations were statistically significant.
Kinetics of Change in Cellular/Tissue HIV-1 DNA
There was no evidence of consistent decay in tissue HIV-1 DNA for the 15 subjects evaluated for up to a year. When calculated from values in all the subjects, the trajectory slope for the group did not differ from zero. The average decay slope for all 15 subjects was
-0.13 log10 copies/year in gut and -0.16 log10 copies/year in PBMCs (average t 1/2 of 123 weeks in gut and 98 weeks in PBMCs). We could separate the subjects into 3 groups: 3 subjects with undetectable HIV-1 RNA in all compartments at all times (group A), 6 subjects with RNA only in mucosa or PBMCs (group B), and 6 subjects with episodic plasma blips of RNA (group C). Separate analysis of these 3 groups also did not yield any statistical evidence for HIV-1 DNA decay in mucosa and PBMCs (Fig. 1; Table 1).
Quantification of GALT Reservoirs
Because GALT is clearly a source of HIV-1 DNA, we used our data to calculate the number of GALT lymphocytes, separate from blood and lymph nodes, comprising an anatomically distinct reservoir of potentially replication-competent HIV-1. These calculations rest on our previously published, detailed demonstration of reproducibility (from different biopsies at same level in the same subject), sensitivity with minimal intra-assay or intrasubject variability in the method used here to measure mucosal HIV-1 DNA.4 The calculation of "total gut mucosal viral burden" begins with the generally accepted assumptions that CD4+/CD45+RO+ lymphocytes are the major form throughout GALT7-9,3 and are nearly all "activated," depending on the definition used.8,10,11 Importantly, there are few resting memory T cells in this compartment. We also assume that these CD4+/CD45+RO+lymphocytes are uniformly susceptible to HIV-1 infection and that each infected cell contains at least a single, integrated copy of HIV-1 DNA. The work of others suggests that only 1% of HIV-1 DNA in resting CD4 T cells in other compartments is potentially replication competent.12
Based on standard anatomical assumptions about the human GI tract (the small intestine is approximately 5.5 m in length and has a diameter of 2.5 cm; the colon is approximately 1.6 m in length and has a diameter of 7.5 cm), we calculated a value of 671,000 mm3 for the mucosal volume of these 2 organs (including the lamina propria). The biopsy forceps used produced a tissue sample with an average volume of 22.5 mm3; the "mucosal volume" of the GI tract would equate to 29,848 biopsies. The total number of cells in each biopsy was calculated to be approximately 15 million, based on ß-globin DNA measurements in the described samples. This value, multiplied by the total number of biopsies in the mucosa mentioned previously, yields a total of 4.48 X 1011 cells in the mucosa (epithelia and lamina propria) of the small and large intestines. Of these, a commonly accepted proportion of 10% can be assumed to be leukocytes. This is a very conservative estimate, supported by direct measurements of percent lymphocytes in the studied samples (which were acquired from the descending colon). It is known that the ascending colon has more lymphocytes per unit area than the left and small intestine numbers exceed those in the colon. It does not include intensive localizations in lymphoid granules or Peyer patches.
The median value of GALT HIV-1 DNA from our subjects was 36 copies per 2 X 106 ß-globin copies (36 copies per million cells). Thus, after correcting for nonfunctional HIV-1 DNA copies, as suggested previously, there were approximately 160,000 GALT cells with potentially replicative HIV-1 DNA in each of our well-suppressed subjects. The relative importance of this pool can be evaluated by performing the same calculations for the simultaneously measured PBMCs. Peripheral blood mononuclear cells HIV-1 and ß-globin DNA yielded a median value of 354 copies of HIV-1 per 106 PBMCs from all subjects. In an estimated blood volume of 5 L, after correcting for nonfunctional DNA, there were 3.5 potentially replicative HIV-1-containing cells per million PBMCs or a total circulating load of 70,000 cells. Chun et al13 have reported a value of 5 cells per million PBMCs.
METHODS
Study Subjects
The study was reviewed and approved by the University of California, Los Angeles and AIDS Research Alliance institutional review boards, and informed consent was obtained from all the subjects. Fifteen HIV-1-positive subjects (14 male subjects) were recruited. All met the following criteria: plasma HIV-1 RNA of less than 50 copies/mL (using the Roche Amplicor ultrasensitive assay [Roche Diagnostics, Indianapolis, IN]) upon entry, with documented levels of 200 copies/mL for at least 3 months before and self-reported undetectable levels for at least 12 months before enrollment; HAART (defined as ≥2 antiretroviral medications, including a protease inhibitor) had been received for at least 24 months before enrollment. Subject characteristics recorded included sex, age, Centers for Disease Control and Prevention class, CD4 T-cell count, months of known HIV-1 infection, time between diagnosis and initiation of HAART, and duration of HAART.
Samples and Schedule
Endoscopic biopsies of colonic mucosa were obtained during flexible sigmoidoscopy. Biopsies were taken at a standardized site in the rectosigmoid region 30 cm from the anal margin using large-cup endoscopic biopsy forceps (outside diameter of 3.3 mm; Microvasive Radial Jaw; Boston Scientific, Boston, MA). One biopsy specimen was immediately frozen in liquid nitrogen for later analysis of DNA and RNA, and tissue was collected at the same level for histopathologic assessment. Using routine hematoxylin and eosin staining, tissue was examined for confounding conditions such as inflammation secondary to infectious or traumatic proctitis. Blood was drawn immediately before endoscopy into EDTA-containing tubes. The PBMCs were prepared by centrifugation on a Ficoll-Hypaque density gradient (Nycomed Pharma AS, Oslo, Norway). These procedures were repeated every 3 months for a total of 12 months.
Analyses
Histologic review was conducted by a dedicated GI pathologist (Dr G. Cortina from the University of California, Los Angeles). Individual endoscopic biopsies and isolated PBMCs were kept frozen at -80 C until analysis of nucleic acids. Nucleic acid extraction and quantification were performed according to our previously described methods, which demonstrates negligible intrasubject variability in HIV RNA and DNA yields from each of the multiple biopsies at the same level in the colon in the same subject; the lower limit of detection for HIV-1 RNA was 10 copies/mg total RNA, and that for HIV-1 DNA was 10 copies/2 X 106 copies of ß-globin.4 For statistical assessment, log-transformed measurements of HIV-1 DNA and RNA were modeled using mixed-effect models.5,6 The model used assumes subject-specific linear trajectories. Individual values were assumed to be from a normally distributed population. Values below the limit of detectability for an assay were assumed to be left censored at the assay limit. To account for censoring and to make the best use of the relatively sparse data, a Bayesian framework was used for the fitting using the WinBUGS software package (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK).2 Markov chain Monte Carlo simulations from the posterior distribution for the slope parameters were used to perform inferences about decay and half-life parameters for individual subjects.
|
|
| |
| |
|
|
|